Function and Regulation of Nuclear DNA Sensors During Viral Infection and Tumorigenesis
- PMID: 33505405
- PMCID: PMC7829187
- DOI: 10.3389/fimmu.2020.624556
Function and Regulation of Nuclear DNA Sensors During Viral Infection and Tumorigenesis
Abstract
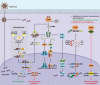
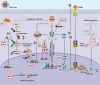
IFI16, hnRNPA2B1, and nuclear cGAS are nuclear-located DNA sensors that play important roles in initiating host antiviral immunity and modulating tumorigenesis. IFI16 triggers innate antiviral immunity, inflammasome, and suppresses tumorigenesis by recognizing double-stranded DNA (dsDNA), single-stranded DNA (ssDNA), damaged nuclear DNA, or cooperatively interacting with multiple tumor suppressors such as p53 and BRCA1. hnRNPA2B1 initiates interferon (IFN)-α/β production and enhances STING-dependent cytosolic antiviral signaling by directly binding viral dsDNA from invaded viruses and facilitating N6 -methyladenosine (m6A) modification of cGAS, IFI16, and STING mRNAs. Nuclear cGAS is recruited to double-stranded breaks (DSBs), suppresses DNA repair, and promotes tumorigenesis. This review briefly describes the nuclear functions of IFI16, hnRNPA2B1, and cGAS, and summarizes the transcriptional, post-transcriptional, and post-translational regulation of these nuclear DNA sensors.
Keywords: IFI16; cGAS; hnRNPA2B1; nuclear DNA sensor; p53; tumorigenesis; type I interferon.
Copyright © 2021 Zhang, Yuan and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Nuclear hnRNPA2B1 initiates and amplifies the innate immune response to DNA viruses.Science. 2019 Aug 16;365(6454):eaav0758. doi: 10.1126/science.aav0758. Epub 2019 Jul 18. Science. 2019. PMID: 31320558
-
The intracellular DNA sensors cGAS and IFI16 do not mediate effective antiviral immune responses to HSV-1 in human microglial cells.J Neurovirol. 2020 Aug;26(4):544-555. doi: 10.1007/s13365-020-00852-1. Epub 2020 Jun 2. J Neurovirol. 2020. PMID: 32488842 Free PMC article.
-
Viral DNA Sensors IFI16 and Cyclic GMP-AMP Synthase Possess Distinct Functions in Regulating Viral Gene Expression, Immune Defenses, and Apoptotic Responses during Herpesvirus Infection.mBio. 2016 Nov 15;7(6):e01553-16. doi: 10.1128/mBio.01553-16. mBio. 2016. PMID: 27935834 Free PMC article.
-
The emerging role of nuclear viral DNA sensors.J Biol Chem. 2015 Oct 30;290(44):26412-21. doi: 10.1074/jbc.R115.652289. Epub 2015 Sep 9. J Biol Chem. 2015. PMID: 26354430 Free PMC article. Review.
-
The interferon-inducible DNA-sensor protein IFI16: a key player in the antiviral response.New Microbiol. 2015 Jan;38(1):5-20. Epub 2015 Jan 1. New Microbiol. 2015. PMID: 25742143 Review.
Cited by
-
Viral Manipulation of the Host Epigenome as a Driver of Virus-Induced Oncogenesis.Microorganisms. 2021 May 30;9(6):1179. doi: 10.3390/microorganisms9061179. Microorganisms. 2021. PMID: 34070716 Free PMC article. Review.
-
IFI16 Induced by Direct Interaction between Esophageal Squamous Cell Carcinomas and Macrophages Promotes Tumor Progression via Secretion of IL-1α.Cells. 2023 Nov 10;12(22):2603. doi: 10.3390/cells12222603. Cells. 2023. PMID: 37998338 Free PMC article.
-
Sirt1 Negatively Regulates Cellular Antiviral Responses by Preventing the Cytoplasmic Translocation of Interferon-Inducible Protein 16 in Human Cells.J Virol. 2023 Feb 28;97(2):e0197522. doi: 10.1128/jvi.01975-22. Epub 2023 Feb 7. J Virol. 2023. PMID: 36749073 Free PMC article.
-
m6A reader proteins: the executive factors in modulating viral replication and host immune response.Front Cell Infect Microbiol. 2023 May 30;13:1151069. doi: 10.3389/fcimb.2023.1151069. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37325513 Free PMC article. Review.
-
METTL14 Induced N6-Methyladenosine Modification of FOXP4 mRNA in HBV-HCC.J Cancer. 2024 Oct 14;15(19):6232-6238. doi: 10.7150/jca.101385. eCollection 2024. J Cancer. 2024. PMID: 39513116 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

