Anti-Müllerian hormone (AMH) autocrine signaling promotes survival and proliferation of ovarian cancer cells
- PMID: 33500516
- PMCID: PMC7838181
- DOI: 10.1038/s41598-021-81819-y
Anti-Müllerian hormone (AMH) autocrine signaling promotes survival and proliferation of ovarian cancer cells
Abstract
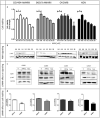
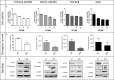
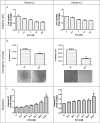
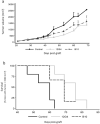
In ovarian carcinoma, anti-Müllerian hormone (AMH) type II receptor (AMHRII) and the AMH/AMHRII signaling pathway are potential therapeutic targets. Here, AMH dose-dependent effect on signaling and proliferation was analyzed in four ovarian cancer cell lines, including sex cord stromal/granulosa cell tumors and high grade serous adenocarcinomas (COV434-AMHRII, SKOV3-AMHRII, OVCAR8 and KGN). As previously shown, incubation with exogenous AMH at concentrations above the physiological range (12.5-25 nM) decreased cell viability. Conversely, physiological concentrations of endogenous AMH improved cancer cell viability. Partial AMH depletion by siRNAs was sufficient to reduce cell viability in all four cell lines, by 20% (OVCAR8 cells) to 40% (COV434-AMHRII cells). In the presence of AMH concentrations within the physiological range (5 to 15 pM), the newly developed anti-AMH B10 antibody decreased by 25% (OVCAR8) to 50% (KGN) cell viability at concentrations ranging between 3 and 333 nM. At 70 nM, B10 reduced clonogenic survival by 57.5%, 57.1%, 64.7% and 37.5% in COV434-AMHRII, SKOV3-AMHRII, OVCAR8 and KGN cells, respectively. In the four cell lines, B10 reduced AKT phosphorylation, and increased PARP and caspase 3 cleavage. These results were confirmed in ovarian cancer cells isolated from patients' ascites, demonstrating the translational potential of these results. Furthermore, B10 reduced COV434-MISRII tumor growth in vivo and significantly enhanced the median survival time compared with vehicle (69 vs 60 days; p = 0.0173). Our data provide evidence for a novel pro-survival autocrine role of AMH in the context of ovarian cancer, which was targeted therapeutically using an anti-AMH antibody to successfully repress tumor growth.
Conflict of interest statement
Maëva Chauvin, Myriam Chentouf, Pierre Martineau, Bruno Robert, and André Pèlegrin are inventors of a patent “ANTI-MÜLLERIAN INHIBITING SUBSTANCE ANTIBODIES AND USES THEREOF” filed on 2019, September, 27. Maëva Chauvin, Thierry Chardès, Isabelle Navarro-Teulon and André Pèlegrin are inventors of a patent “USE OF MÜLLERIAN INHIBITING SUBSTANCE INHIBITORS FOR TREATING CANCER” filed on 2019, September, 27. David Pépin is inventor of the patent “MODIFIED MULLERIAN INHIBITING SUBSTANCE (MIS) PROTEINS AND USES THEREOF FOR THE TREATMENT OF DISEASES”, WO2014164981A1. The other authors declare no competing interests.
Figures
Similar articles
-
Anti-Müllerian hormone concentration regulates activin receptor-like kinase-2/3 expression levels with opposing effects on ovarian cancer cell survival.Int J Oncol. 2021 Jul;59(1):43. doi: 10.3892/ijo.2021.5223. Epub 2021 May 20. Int J Oncol. 2021. PMID: 34013359 Free PMC article.
-
Expression of Müllerian-Inhibiting Substance/Anti-Müllerian Hormone Type II Receptor in the Human Theca Cells.J Clin Endocrinol Metab. 2018 Sep 1;103(9):3376-3385. doi: 10.1210/jc.2018-00549. J Clin Endocrinol Metab. 2018. PMID: 29947765
-
The regulation and signalling of anti-Müllerian hormone in human granulosa cells: relevance to polycystic ovary syndrome.Hum Reprod. 2019 Dec 1;34(12):2467-2479. doi: 10.1093/humrep/dez214. Hum Reprod. 2019. PMID: 31735954
-
The roles of anti-Müllerian hormone in breast cancer.Endocr Relat Cancer. 2023 Aug 14;30(10):e230060. doi: 10.1530/ERC-23-0060. Print 2023 Oct 1. Endocr Relat Cancer. 2023. PMID: 37410375 Free PMC article. Review.
-
Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary.Hum Reprod Update. 2016 Nov;22(6):709-724. doi: 10.1093/humupd/dmw027. Epub 2016 Aug 27. Hum Reprod Update. 2016. PMID: 27566840 Review.
Cited by
-
Bone morphogenetic protein signaling is a possible therapeutic target in gynecologic cancer.Cancer Sci. 2023 Mar;114(3):722-729. doi: 10.1111/cas.15682. Epub 2022 Dec 16. Cancer Sci. 2023. PMID: 36468782 Free PMC article. Review.
-
Anti-Müllerian hormone type II receptor protein expression in non-small cell lung cancer and the effect of AMH/AMHR2 signaling on cancer cell proliferation.Thorac Cancer. 2024 Oct;15(29):2090-2099. doi: 10.1111/1759-7714.15309. Epub 2024 Sep 4. Thorac Cancer. 2024. PMID: 39230026 Free PMC article.
-
Role of transforming growth factor-β in peripheral nerve regeneration.Neural Regen Res. 2024 Feb;19(2):380-386. doi: 10.4103/1673-5374.377588. Neural Regen Res. 2024. PMID: 37488894 Free PMC article. Review.
-
Anti-Müllerian hormone: biology and role in endocrinology and cancers.Front Endocrinol (Lausanne). 2024 Sep 16;15:1468364. doi: 10.3389/fendo.2024.1468364. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39351532 Free PMC article. Review.
-
Blood Lead Level as Marker of Increased Risk of Ovarian Cancer in BRCA1 Carriers.Nutrients. 2024 Apr 30;16(9):1370. doi: 10.3390/nu16091370. Nutrients. 2024. PMID: 38732616 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

