Epitope-resolved profiling of the SARS-CoV-2 antibody response identifies cross-reactivity with endemic human coronaviruses
- PMID: 33495758
- PMCID: PMC7816965
- DOI: 10.1016/j.xcrm.2020.100189
Epitope-resolved profiling of the SARS-CoV-2 antibody response identifies cross-reactivity with endemic human coronaviruses
Abstract
The SARS-CoV-2 proteome shares regions of conservation with endemic human coronaviruses (CoVs), but it remains unknown to what extent these may be cross-recognized by the antibody response. Here, we study cross-reactivity using a highly multiplexed peptide assay (PepSeq) to generate an epitope-resolved view of IgG reactivity across all human CoVs in both COVID-19 convalescent and negative donors. PepSeq resolves epitopes across the SARS-CoV-2 Spike and Nucleocapsid proteins that are commonly targeted in convalescent donors, including several sites also recognized in some uninfected controls. By comparing patterns of homologous reactivity between CoVs and using targeted antibody-depletion experiments, we demonstrate that SARS-CoV-2 elicits antibodies that cross-recognize pandemic and endemic CoV antigens at two Spike S2 subunit epitopes. We further show that these cross-reactive antibodies preferentially bind endemic homologs. Our findings highlight sites at which the SARS-CoV-2 response appears to be shaped by previous CoV exposures and which have the potential to raise broadly neutralizing responses.
Keywords: SARS-CoV-2; antibody response; cross-reactivity; endemic CoVs; highly multiplexed serology.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

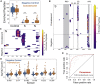


Update of
-
Epitope-resolved profiling of the SARS-CoV-2 antibody response identifies cross-reactivity with an endemic human CoV.bioRxiv [Preprint]. 2020 Jul 27:2020.07.27.222943. doi: 10.1101/2020.07.27.222943. bioRxiv. 2020. Update in: Cell Rep Med. 2021 Jan 19;2(1):100189. doi: 10.1016/j.xcrm.2020.100189 PMID: 32743570 Free PMC article. Updated. Preprint.
Similar articles
-
Epitope-resolved profiling of the SARS-CoV-2 antibody response identifies cross-reactivity with an endemic human CoV.bioRxiv [Preprint]. 2020 Jul 27:2020.07.27.222943. doi: 10.1101/2020.07.27.222943. bioRxiv. 2020. Update in: Cell Rep Med. 2021 Jan 19;2(1):100189. doi: 10.1016/j.xcrm.2020.100189 PMID: 32743570 Free PMC article. Updated. Preprint.
-
COVID-19 vaccination elicits an evolving, cross-reactive antibody response to epitopes conserved with endemic coronavirus spike proteins.Cell Rep. 2022 Jul 5;40(1):111022. doi: 10.1016/j.celrep.2022.111022. Epub 2022 Jun 13. Cell Rep. 2022. PMID: 35753310 Free PMC article.
-
COVID-19 vaccination recruits and matures cross-reactive antibodies to conserved epitopes in endemic coronavirus Spike proteins.medRxiv [Preprint]. 2022 Jan 25:2022.01.24.22269542. doi: 10.1101/2022.01.24.22269542. medRxiv. 2022. Update in: Cell Rep. 2022 Jul 5;40(1):111022. doi: 10.1016/j.celrep.2022.111022 PMID: 35118479 Free PMC article. Updated. Preprint.
-
Immunologic Testing for SARS-CoV-2 Infection from the Antigen Perspective.J Clin Microbiol. 2021 Apr 20;59(5):e02160-20. doi: 10.1128/JCM.02160-20. Print 2021 Apr 20. J Clin Microbiol. 2021. PMID: 33318065 Free PMC article. Review.
-
Unravelling Antigenic Cross-Reactions toward the World of Coronaviruses: Extent of the Stability of Shared Epitopes and SARS-CoV-2 Anti-Spike Cross-Neutralizing Antibodies.Pathogens. 2023 May 13;12(5):713. doi: 10.3390/pathogens12050713. Pathogens. 2023. PMID: 37242383 Free PMC article. Review.
Cited by
-
Clinical symptoms, comorbidities and health outcomes among outpatients infected with the common cold coronaviruses versus influenza virus.Virol J. 2024 Oct 8;21(1):251. doi: 10.1186/s12985-024-02524-6. Virol J. 2024. PMID: 39380036 Free PMC article.
-
Severe acute respiratory syndrome coronavirus 2-reactive salivary antibody detection in South Carolina emergency healthcare workers, September 2019-March 2020.Epidemiol Infect. 2024 Sep 25;152:e102. doi: 10.1017/S0950268824000967. Epidemiol Infect. 2024. PMID: 39320488 Free PMC article.
-
Mapping disparities in viral infection rates using highly multiplexed serology.mSphere. 2024 Sep 25;9(9):e0012724. doi: 10.1128/msphere.00127-24. Epub 2024 Aug 20. mSphere. 2024. PMID: 39162531 Free PMC article.
-
Enhanced Assessment of Cross-Reactive Antigenic Determinants within the Spike Protein.Int J Mol Sci. 2024 Jul 26;25(15):8180. doi: 10.3390/ijms25158180. Int J Mol Sci. 2024. PMID: 39125749 Free PMC article.
-
Passive infusion of an S2-Stem broadly neutralizing antibody protects against SARS-CoV-2 infection and lower airway inflammation in rhesus macaques.bioRxiv [Preprint]. 2024 Jul 30:2024.07.30.605768. doi: 10.1101/2024.07.30.605768. bioRxiv. 2024. PMID: 39109178 Free PMC article. Preprint.
References
-
- Piñana J.L., Xhaard A., Tridello G., Passweg J., Kozijn A., Polverelli N., Heras I., Perez A., Sanz J., Berghuis D., Infectious Diseases Working Party of the European Society for Blood and Marrow Transplantation and Infectious Complications Subcommittee of the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group (GETH) Seasonal human coronaviruses respiratory tract infection in recipients of allogeneic hematopoietic stem cell transplantation. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa553. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

