Mice with cleavage-resistant N-cadherin exhibit synapse anomaly in the hippocampus and outperformance in spatial learning tasks
- PMID: 33494786
- PMCID: PMC7831172
- DOI: 10.1186/s13041-021-00738-1
Mice with cleavage-resistant N-cadherin exhibit synapse anomaly in the hippocampus and outperformance in spatial learning tasks
Abstract
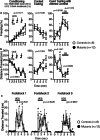
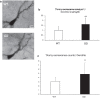
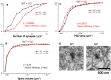
N-cadherin is a homophilic cell adhesion molecule that stabilizes excitatory synapses, by connecting pre- and post-synaptic termini. Upon NMDA receptor (NMDAR) activation by glutamate, membrane-proximal domains of N-cadherin are cleaved serially by a-disintegrin-and-metalloprotease 10 (ADAM10) and then presenilin 1(PS1, catalytic subunit of the γ-secretase complex). To assess the physiological significance of the initial N-cadherin cleavage, we engineer the mouse genome to create a knock-in allele with tandem missense mutations in the mouse N-cadherin/Cadherin-2 gene (Cdh2 R714G, I715D, or GD) that confers resistance on proteolysis by ADAM10 (GD mice). GD mice showed a better performance in the radial maze test, with significantly less revisiting errors after intervals of 30 and 300 s than WT, and a tendency for enhanced freezing in fear conditioning. Interestingly, GD mice reveal higher complexity in the tufts of thorny excrescence in the CA3 region of the hippocampus. Fine morphometry with serial section transmission electron microscopy (ssTEM) and three-dimensional (3D) reconstruction reveals significantly higher synaptic density, significantly smaller PSD area, and normal dendritic spine volume in GD mice. This knock-in mouse has provided in vivo evidence that ADAM10-mediated cleavage is a critical step in N-cadherin shedding and degradation and involved in the structure and function of glutamatergic synapses, which affect the memory function.
Keywords: ADAM10; Hippocampus; N-cadherin; Synapse; Working memory.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Characterization of sequential N-cadherin cleavage by ADAM10 and PS1.Neurosci Lett. 2006 Jul 24;402(3):278-83. doi: 10.1016/j.neulet.2006.04.018. Epub 2006 May 9. Neurosci Lett. 2006. PMID: 16687212
-
Amyloid beta inhibits ectodomain shedding of N-cadherin via down-regulation of cell-surface NMDA receptor.Neuroscience. 2007 Mar 2;145(1):5-10. doi: 10.1016/j.neuroscience.2006.12.022. Epub 2007 Jan 25. Neuroscience. 2007. PMID: 17257767
-
Defective synaptic transmission and structure in the dentate gyrus and selective fear memory impairment in the Rsk2 mutant mouse model of Coffin-Lowry syndrome.Neurobiol Dis. 2013 Oct;58:156-68. doi: 10.1016/j.nbd.2013.05.016. Epub 2013 Jun 3. Neurobiol Dis. 2013. PMID: 23742761
-
PLD1 promotes dendritic spine development by inhibiting ADAM10-mediated N-cadherin cleavage.Sci Rep. 2017 Jul 20;7(1):6035. doi: 10.1038/s41598-017-06121-2. Sci Rep. 2017. PMID: 28729535 Free PMC article.
-
Postnatal disruption of the disintegrin/metalloproteinase ADAM10 in brain causes epileptic seizures, learning deficits, altered spine morphology, and defective synaptic functions.J Neurosci. 2013 Aug 7;33(32):12915-28, 12928a. doi: 10.1523/JNEUROSCI.5910-12.2013. J Neurosci. 2013. PMID: 23926248 Free PMC article.
Cited by
-
Plk2 promotes synaptic destabilization through disruption of N-cadherin adhesion complexes during homeostatic adaptation to hyperexcitation.J Neurochem. 2023 Nov;167(3):362-375. doi: 10.1111/jnc.15948. Epub 2023 Aug 31. J Neurochem. 2023. PMID: 37654026 Free PMC article.
-
Neuroprotection by ADAM10 inhibition requires TrkB signaling in the Huntington's disease hippocampus.Cell Mol Life Sci. 2024 Aug 7;81(1):333. doi: 10.1007/s00018-024-05382-1. Cell Mol Life Sci. 2024. PMID: 39112663 Free PMC article.
-
Flying under the radar: CDH2 (N-cadherin), an important hub molecule in neurodevelopmental and neurodegenerative diseases.Front Neurosci. 2022 Sep 23;16:972059. doi: 10.3389/fnins.2022.972059. eCollection 2022. Front Neurosci. 2022. PMID: 36213737 Free PMC article. Review.
-
Activation of feedforward wiring in adult hippocampal neurons by the basic-helix-loop-helix transcription factor Ascl4.PNAS Nexus. 2024 May 3;3(5):pgae174. doi: 10.1093/pnasnexus/pgae174. eCollection 2024 May. PNAS Nexus. 2024. PMID: 38711810 Free PMC article.
-
Tau beyond Tangles: DNA Damage Response and Cytoskeletal Protein Crosstalk on Neurodegeneration.Int J Mol Sci. 2024 Jul 19;25(14):7906. doi: 10.3390/ijms25147906. Int J Mol Sci. 2024. PMID: 39063148 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

