GlycoBODIPYs: Sugars Serving as a Natural Stock for Water-soluble Fluorescent Probes of Complex Chiral Morphology
- PMID: 33492705
- PMCID: PMC8048574
- DOI: 10.1002/anie.202016764
GlycoBODIPYs: Sugars Serving as a Natural Stock for Water-soluble Fluorescent Probes of Complex Chiral Morphology
Abstract
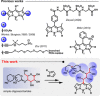
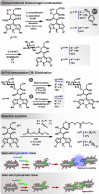
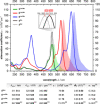
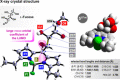
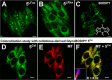
A range of unprocessed, reducing sugar substrates (mono-, di-, and trisaccharides) is shown to take part in a straightforward four-step synthetic route to water-soluble, uncharged BODIPY derivatives with unimpaired chiral integrity and high fluorescence efficiency. A wide compatibility with several postfunctionalizations is demonstrated, thus suggesting a universal utility of the multifunctional glycoconjugates, which we call GlycoBODIPYs. Knoevenagel condensations are able to promote a red-shift in the spectra, thereby furnishing strongly fluorescent red and far-red glycoconjugates of high hydrophilicity. The synthetic outcome was studied by X-ray crystallography and by comprehensive photophysical investigations in several solvent systems. Furthermore, cell experiments illustrate efficient cell uptake and demonstrate differential cell targeting as a function of the integrated chiral information.
Keywords: carbohydrates; cell imaging; dyes; fluorophores; water solubility.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Water-soluble phosphonate-substituted BODIPY derivatives with tunable emission channels.Org Lett. 2011 Jun 17;13(12):3072-5. doi: 10.1021/ol200969r. Epub 2011 May 20. Org Lett. 2011. PMID: 21598985
-
BODIPYs as Chemically Stable Fluorescent Tags for Synthetic Glycosylation Strategies towards Fluorescently Labeled Saccharides.Chemistry. 2020 Apr 24;26(24):5388-5399. doi: 10.1002/chem.201905780. Epub 2020 Mar 13. Chemistry. 2020. PMID: 31999023
-
Highly water-soluble neutral BODIPY dyes with controllable fluorescence quantum yields.Org Lett. 2011 Feb 4;13(3):438-41. doi: 10.1021/ol102758z. Epub 2010 Dec 22. Org Lett. 2011. PMID: 21175151 Free PMC article.
-
Bringing Color to Sugars: The Chemical Assembly of Carbohydrates to BODIPY Dyes.Chem Rec. 2021 Nov;21(11):3112-3130. doi: 10.1002/tcr.202100190. Epub 2021 Sep 2. Chem Rec. 2021. PMID: 34472184 Review.
-
Bioapplications of small molecule Aza-BODIPY: from rational structural design to in vivo investigations.Chem Soc Rev. 2020 Nov 7;49(21):7533-7567. doi: 10.1039/d0cs00234h. Epub 2020 Sep 30. Chem Soc Rev. 2020. PMID: 32996497 Review.
Cited by
-
De Novo Access to BODIPY C-Glycosides as Linker-Free Nonsymmetrical BODIPY-Carbohydrate Conjugates.J Org Chem. 2024 Mar 15;89(6):4042-4055. doi: 10.1021/acs.joc.3c02907. Epub 2024 Mar 4. J Org Chem. 2024. PMID: 38438277 Free PMC article.
-
Transforming Dyes into Fluorophores: Exciton-Induced Emission with Chain-like Oligo-BODIPY Superstructures.Angew Chem Int Ed Engl. 2022 May 23;61(22):e202116834. doi: 10.1002/anie.202116834. Epub 2022 Mar 16. Angew Chem Int Ed Engl. 2022. PMID: 35244983 Free PMC article.
-
A Multifunctional AIE Nanoprobe as a Drug Delivery Bioimaging and Cancer Treatment System.Front Bioeng Biotechnol. 2021 Nov 8;9:766470. doi: 10.3389/fbioe.2021.766470. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34820365 Free PMC article.
-
DNA-Programmed Lipid Nanoreactors for Synthesis of Carbohydrate Mimetics by Fusion of Aqueous Sub-attoliter Compartments.J Am Chem Soc. 2023 Sep 13;145(36):19633-19641. doi: 10.1021/jacs.3c04093. Epub 2023 Aug 24. J Am Chem Soc. 2023. PMID: 37619973 Free PMC article.
References
-
- None
-
- Treibs A., Kreuzer F.-H., Justus Liebigs Ann. Chem. 1968, 718, 208; - PubMed
-
- Loudet A., Burgess K., Chem. Rev. 2007, 107, 4891; - PubMed
-
- Ulrich G., Ziessel R., Harriman A., Angew. Chem. Int. Ed. 2008, 47, 1184; - PubMed
- Angew. Chem. 2008, 120, 1202;
-
- Glembockyte V., Frenette M., Mottillo C., Durantini A. M., Gostick J., Štrukil V., Friščić T., Cosa G., J. Am. Chem. Soc. 2018, 140, 16882; - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

