A Novel Triple-Fluorescent HCMV Strain Reveals Gene Expression Dynamics and Anti-Herpesviral Drug Mechanisms
- PMID: 33489928
- PMCID: PMC7820782
- DOI: 10.3389/fcimb.2020.536150
A Novel Triple-Fluorescent HCMV Strain Reveals Gene Expression Dynamics and Anti-Herpesviral Drug Mechanisms
Abstract
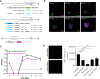
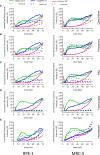
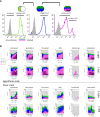
Human Cytomegalovirus (HCMV) infection may result in severe outcomes in immunocompromised individuals such as AIDS patients, transplant recipients, and neonates. To date, no vaccines are available and there are only few drugs for anti-HCMV therapy. Adverse effects and the continuous emergence of drug-resistance strains require the identification of new drug candidates in the near future. Identification and characterization of such compounds and biological factors requires sensitive and reliable detection techniques of HCMV infection, gene expression and spread. In this work, we present and validate a novel concept for multi-reporter herpesviruses, identified through iterative testing of minimally invasive mutations. We integrated up to three fluorescence reporter genes into replication-competent HCMV strains, generating reporter HCMVs that allow the visualization of replication cycle stages of HCMV, namely the immediate early (IE), early (E), and late (L) phase. Fluorescent proteins with clearly distinguishable emission spectra were linked by 2A peptides to essential viral genes, allowing bicistronic expression of the viral and the fluorescent protein without major effects on viral fitness. By using this triple color reporter HCMV, we monitored gene expression dynamics of the IE, E, and L genes by measuring the fluorescent signal of the viral gene-associated fluorophores within infected cell populations and at high temporal resolution. We demonstrate distinct inhibitory profiles of foscarnet, fomivirsen, phosphonoacetic acid, ganciclovir, and letermovir reflecting their mode-of-action. In conclusion, our data argues that this experimental approach allows the identification and characterization of new drug candidates in a single step.
Keywords: Human Cytomegalovirus; antivirals; herpesvirus; in vitro drug testing; letermovir; live-cell imaging; reporter assay; screening.
Copyright © 2021 Rand, Kubsch, Kasmapour and Cicin-Sain.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
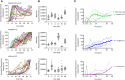
Similar articles
-
In vitro drug combination studies of Letermovir (AIC246, MK-8228) with approved anti-human cytomegalovirus (HCMV) and anti-HIV compounds in inhibition of HCMV and HIV replication.Antimicrob Agents Chemother. 2015;59(6):3140-8. doi: 10.1128/AAC.00114-15. Epub 2015 Mar 16. Antimicrob Agents Chemother. 2015. PMID: 25779572 Free PMC article.
-
Novel cytomegalovirus-inhibitory compounds of the class pyrrolopyridines show a complex pattern of target binding that suggests an unusual mechanism of antiviral activity.Antiviral Res. 2018 Nov;159:84-94. doi: 10.1016/j.antiviral.2018.09.012. Epub 2018 Sep 27. Antiviral Res. 2018. PMID: 30268914
-
Inhibitors of dual-specificity tyrosine phosphorylation-regulated kinases (DYRK) exert a strong anti-herpesviral activity.Antiviral Res. 2017 Jul;143:113-121. doi: 10.1016/j.antiviral.2017.04.003. Epub 2017 Apr 9. Antiviral Res. 2017. PMID: 28400201
-
Drug targets in cytomegalovirus infection.Infect Disord Drug Targets. 2009 Apr;9(2):201-22. doi: 10.2174/187152609787847758. Infect Disord Drug Targets. 2009. PMID: 19275707 Review.
-
The discovery and development of filociclovir for the prevention and treatment of human cytomegalovirus-related disease.Antiviral Res. 2020 Apr;176:104710. doi: 10.1016/j.antiviral.2020.104710. Epub 2020 Jan 12. Antiviral Res. 2020. PMID: 31940473 Review.
Cited by
-
Foscarnet-Type Inorganic-Organic Hybrid Nanoparticles for Effective Antiviral Therapy.ACS Biomater Sci Eng. 2022 Apr 11;8(4):1596-1603. doi: 10.1021/acsbiomaterials.2c00074. Epub 2022 Mar 28. ACS Biomater Sci Eng. 2022. PMID: 35344659 Free PMC article.
-
Dynamic monitoring of viral gene expression reveals rapid antiviral effects of CD8 T cells recognizing the HCMV-pp65 antigen.Front Immunol. 2024 Jul 15;15:1439184. doi: 10.3389/fimmu.2024.1439184. eCollection 2024. Front Immunol. 2024. PMID: 39104541 Free PMC article.
-
DNA methylation profiling identifies TBKBP1 as potent amplifier of cytotoxic activity in CMV-specific human CD8+ T cells.PLoS Pathog. 2024 Sep 26;20(9):e1012581. doi: 10.1371/journal.ppat.1012581. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39325839 Free PMC article.
-
Computational modeling of protracted HCMV replication using genome substrates and protein temporal profiles.Proc Natl Acad Sci U S A. 2022 Aug 30;119(35):e2201787119. doi: 10.1073/pnas.2201787119. Epub 2022 Aug 22. Proc Natl Acad Sci U S A. 2022. PMID: 35994667 Free PMC article.
-
Human cytomegalovirus exploits STING signaling and counteracts IFN/ISG induction to facilitate infection of dendritic cells.Nat Commun. 2024 Feb 26;15(1):1745. doi: 10.1038/s41467-024-45614-3. Nat Commun. 2024. PMID: 38409141 Free PMC article.
References
-
- Azad R. F., Driver V. B., Tanaka K., Crooke R. M., Anderson K. P. (1993). Antiviral activity of a phosphorothioate oligonucleotide complementary to RNA of the human cytomegalovirus major immediate-early region. Antimicrob. Agents Chemother. 37 (9), 1945–1954. 10.1128/aac.37.9.1945 - DOI - PMC - PubMed
-
- Boppana S., WJ B. (2013). “Synopsis of Clinical Aspects of Human Cytomegalovirus Disease,” in Cytomegaloviruses: From Molecular Pathogenesis to Intervention. Ed. Reddehase M. J. (Norfol, UK: Caister Academic Press; ), 1–26.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

