Diagnostic Accuracy of the Panbio Severe Acute Respiratory Syndrome Coronavirus 2 Antigen Rapid Test Compared with Reverse-Transcriptase Polymerase Chain Reaction Testing of Nasopharyngeal Samples in the Pediatric Population
- PMID: 33484697
- PMCID: PMC7826137
- DOI: 10.1016/j.jpeds.2021.01.027
Diagnostic Accuracy of the Panbio Severe Acute Respiratory Syndrome Coronavirus 2 Antigen Rapid Test Compared with Reverse-Transcriptase Polymerase Chain Reaction Testing of Nasopharyngeal Samples in the Pediatric Population
Abstract
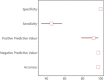
We conducted a multicenter clinical validity study of the Panbio coronavirus disease 2019 Antigen Rapid Test of nasopharyngeal samples in pediatric patients with coronavirus disease 2019-compatible symptoms of ≤5 days of evolution. Our study showed limited accuracy in nasopharyngeal antigen testing: overall sensitivity was 45.4%, and 99.8% of specificity, positive-predictive value was 92.5%.
Keywords: COVID-19; PCR; SARS-CoV-2; antigen test.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
Comment in
-
Diagnostic performance of antigen testing for severe acute respiratory syndrome coronavirus 2.J Pediatr. 2021 Jun;233:283. doi: 10.1016/j.jpeds.2021.02.052. Epub 2021 Feb 22. J Pediatr. 2021. PMID: 33631167 Free PMC article. No abstract available.
-
Reply.J Pediatr. 2021 Jun;233:283-284. doi: 10.1016/j.jpeds.2021.02.051. Epub 2021 Feb 22. J Pediatr. 2021. PMID: 33631168 Free PMC article. No abstract available.
Similar articles
-
Clinical Evaluation of the Accuracy of the Panbio™ COVID-19/Flu A&B Rapid Panel: A Combination Antigen Rapid Diagnostic Test for the Omicron Variant and Influenza A Virus.Viral Immunol. 2024 Aug;37(6):317-321. doi: 10.1089/vim.2024.0039. Epub 2024 Jul 13. Viral Immunol. 2024. PMID: 39001845
-
Evaluation of clinical utility of novel coronavirus antigen detection reagent, Espline® SARS-CoV-2.J Infect Chemother. 2021 Feb;27(2):319-322. doi: 10.1016/j.jiac.2020.11.015. Epub 2020 Dec 23. J Infect Chemother. 2021. PMID: 33388232 Free PMC article.
-
Field evaluation of COVID-19 antigen tests versus RNA based detection: Potential lower sensitivity compensated by immediate results, technical simplicity, and low cost.J Med Virol. 2021 Jul;93(7):4405-4410. doi: 10.1002/jmv.26985. Epub 2021 Apr 8. J Med Virol. 2021. PMID: 33788270 Free PMC article.
-
Immunochromatographic test for the detection of SARS-CoV-2 in saliva.J Infect Chemother. 2021 Feb;27(2):384-386. doi: 10.1016/j.jiac.2020.11.016. Epub 2020 Dec 23. J Infect Chemother. 2021. PMID: 33397587 Free PMC article.
-
Current status of the lateral flow immunoassay for the detection of SARS-CoV-2 in nasopharyngeal swabs.Biochem Med (Zagreb). 2021 Jun 15;31(2):020601. doi: 10.11613/BM.2021.020601. Biochem Med (Zagreb). 2021. PMID: 34140830 Free PMC article. Review.
Cited by
-
Prospective evaluation of the point-of-care use of a rapid antigenic SARS-CoV-2 immunochromatographic test in a paediatric emergency department.Clin Microbiol Infect. 2022 May;28(5):734.e1-734.e6. doi: 10.1016/j.cmi.2021.12.019. Epub 2022 Jan 20. Clin Microbiol Infect. 2022. PMID: 35065265 Free PMC article.
-
Flu@home: the Comparative Accuracy of an At-Home Influenza Rapid Diagnostic Test Using a Prepositioned Test Kit, Mobile App, Mail-in Reference Sample, and Symptom-Based Testing Trigger.J Clin Microbiol. 2022 Mar 16;60(3):e0207021. doi: 10.1128/JCM.02070-21. Epub 2022 Feb 2. J Clin Microbiol. 2022. PMID: 35107302 Free PMC article. Review.
-
Diagnostic accuracy of rapid point-of-care tests for diagnosis of current SARS-CoV-2 infections in children: a systematic review and meta-analysis.BMJ Evid Based Med. 2022 Oct;27(5):274-287. doi: 10.1136/bmjebm-2021-111828. Epub 2022 Jan 18. BMJ Evid Based Med. 2022. PMID: 35042748 Free PMC article.
-
Application of a SARS-CoV-2 Antigen Rapid Immunoassay Based on Active Microfluidic Technology in a Setting of Children and Young Adults.Viruses. 2023 Dec 26;16(1):41. doi: 10.3390/v16010041. Viruses. 2023. PMID: 38257741 Free PMC article.
-
Performance of Antigen Detection Tests for SARS-CoV-2: A Systematic Review and Meta-Analysis.Diagnostics (Basel). 2022 Jun 4;12(6):1388. doi: 10.3390/diagnostics12061388. Diagnostics (Basel). 2022. PMID: 35741198 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention. National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. Overview of Testing for SARS-CoV-2 (COVID-19). Updated October 21, 2020. [Internet]. https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html
-
- Centers for Disease Control and Prevention. National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. Information for Laboratories about Coronavirus (COVID-19). Updated October 16, 2020. [Internet]. https://www.cdc.gov/coronavirus/2019-ncov/lab/index.html
-
- Albert E., Torres I., Bueno F., Huntley D., Moya E., Fernandez-Funtes M.A., et al. Field evaluation of a rapid antigen test (Panbio COVID-19 Ag Rapid Test Device) for the diagnosis of COVID-19 in primary healthcare centers. medRxiv. 2020 http://medrxiv.org/content/early/2020/10/20/2020.10.16.20213850.abstract [Internet]. Accessed December 1, 2020. pre-print(96):2020.10.16.20213850. - PMC - PubMed
-
- Subirana I., Sanz H., Vila J. Building Bivariate tables: the compareGroups package for R. J Stat Softw. 2014;57:11–16.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous