Cost-effectiveness of TLC-NOSF dressings versus neutral dressings for the treatment of diabetic foot ulcers in France
- PMID: 33481840
- PMCID: PMC7822547
- DOI: 10.1371/journal.pone.0245652
Cost-effectiveness of TLC-NOSF dressings versus neutral dressings for the treatment of diabetic foot ulcers in France
Abstract
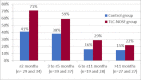
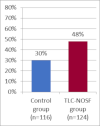
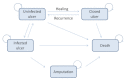
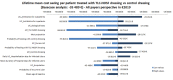
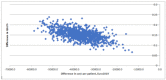
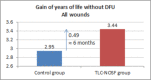
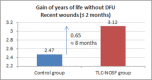
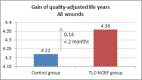
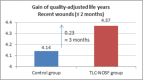
This study assesses the cost-effectiveness of Technology Lipido-Colloid with Nano Oligo Saccharide Factor (TLC-NOSF) wound dressings versus neutral dressings in the management of diabetic foot ulcers (DFUs) from a French collective perspective. We used a Markov microsimulation cohort model to simulate the DFU monthly progression over the lifetime horizon. Our study employed a mixed method design with model inputs including data from interventional and observational studies, French databases and expert opinion. The demographic characteristics of the simulated population and clinical efficacy were based on the EXPLORER double-blind randomized controlled trial. Health-related quality of life, costs, and resource use inputs were taken from the literature relevant to the French context. The main outcomes included life-years without DFU (LYsw/DFU), quality-adjusted life-years (QALYs), amputations, and lifetime costs. To assess the robustness of the results, sensitivity and subgroup analyses based on the wound duration at treatment initiation were performed. Treatment with the TLC-NOSF dressing led to total cost savings per patient of EUR 35,489, associated with gains of 0.50 LYw/DFU and 0.16 QALY. TLC-NOSF dressings were established as the dominant strategy in the base case and all sensitivity analyses. Furthermore, the model revealed that, for every 100 patients treated with TLC-NOSF dressings, two amputations could be avoided. According to the subgroup analysis results, the sooner the TLC-NOSF treatment was initiated, the better were the outcomes, with the highest benefits for ulcers with a duration of two months or less (+0.65 LYw/DFU, +0.23 QALY, and cost savings of EUR 55,710). The results from the French perspective are consistent with the ones from the German and British perspectives. TLC-NOSF dressings are cost-saving compared to neutral dressings, leading to an increase in patients' health benefits and a decrease in the associated treatment costs. These results can thus be used to guide healthcare decisionmakers. The potential savings could represent EUR 3,345 per treated patient per year and even reach EUR 4,771 when TLC-NOSF dressings are used as first line treatment. The EXPLORER trial is registered with ClinicalTrials.gov, number NCT01717183.
Conflict of interest statement
This cost-effectiveness study was funded by Urgo Medical Company FM, https://www.urgo-group.fr/. The assessed dressings are devices marketed by Urgo Medical. The funder provided support in the form of salaries for authors AO, SF, LT, and SB. FM is the CEO of Statesia. A service agreement (commercial contract) was arranged between Urgo Medical and Statesia. Statesia had no other competing interests in the area of this publication relating to employment, consultancy, patents, products in development, or marketed products. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials.
Figures

Similar articles
-
Impact of primary dressings on healing of venous leg ulcers: a French cohort study from the healthcare insurance database.J Wound Care. 2024 Sep 2;33(9):678-686. doi: 10.12968/jowc.2024.0189. J Wound Care. 2024. PMID: 39287032
-
Optimal wound closure of diabetic foot ulcers with early initiation of TLC-NOSF treatment: post-hoc analysis of Explorer.J Wound Care. 2019 Jun 2;28(6):358-367. doi: 10.12968/jowc.2019.28.6.358. J Wound Care. 2019. PMID: 31166858 Clinical Trial.
-
Cost-effectiveness of TLC-sucrose octasulfate versus control dressings in the treatment of diabetic foot ulcers.J Wound Care. 2019 Dec 2;28(12):808-816. doi: 10.12968/jowc.2019.28.12.808. J Wound Care. 2019. PMID: 31825772 Clinical Trial.
-
Uso de apósitos con TLC-NOSF en el manejo de la úlcera de pie diabético, basado en la revisión de la evidencia y la práctica clínica.J Wound Care. 2020 Nov 1;29(LatAm sup 3):31-36. doi: 10.12968/jowc.2020.29.LatAm_sup_3.31. J Wound Care. 2020. PMID: 33251957 Review.
-
Comparing the efficacies of alginate, foam, hydrocolloid, hydrofiber, and hydrogel dressings in the management of diabetic foot ulcers and venous leg ulcers: a systematic review and meta-analysis examining how to dress for success.Dermatol Online J. 2016 Aug 15;22(8):13030/qt7ph5v17z. Dermatol Online J. 2016. PMID: 27617934 Review.
Cited by
-
Role of the Healico© Wound Care Smartphone Application in Preventing a Foot Amputation in a 65-Year-Old Patient with Diabetes.Am J Case Rep. 2022 May 11;23:e936359. doi: 10.12659/AJCR.936359. Am J Case Rep. 2022. PMID: 35538646 Free PMC article.
-
Sucrose Octasulfate-Impregnated Dressings for Adults With Difficult-to-Heal Noninfected Diabetic Foot Ulcers and Difficult-to-Heal Noninfected Venous Leg Ulcers: A Health Technology Assessment.Ont Health Technol Assess Ser. 2024 May 8;24(4):1-101. eCollection 2024. Ont Health Technol Assess Ser. 2024. PMID: 39070301 Free PMC article.
-
A low-cost, antimicrobial aloe-alginate hydrogel film containing Australian First Nations remedy 'lemon myrtle oil' (Backhousia citriodora) - Potential for incorporation into wound dressings.Heliyon. 2024 Sep 12;10(18):e37516. doi: 10.1016/j.heliyon.2024.e37516. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39315217 Free PMC article.
-
Cost-effectiveness of Novel Macrophage-Regulating Treatment for Wound Healing in Patients With Diabetic Foot Ulcers From the Taiwan Health Care Sector Perspective.JAMA Netw Open. 2023 Jan 3;6(1):e2250639. doi: 10.1001/jamanetworkopen.2022.50639. JAMA Netw Open. 2023. PMID: 36633847 Free PMC article. Clinical Trial.
References
-
- World Health Organization. Global report on diabetes; April 2016. [cited 2016 April 4]. Available from: https://www.who.int/publications-detail/global-report-on-diabetes. 10.2337/db15-0956 - DOI - PubMed
-
- Mandereau-Bruno L, Fosse-Edorh S. Prévalence du diabète traité pharmacologiquement (tous types) en France en 2015. Disparités territoriales et socioéconomiques. Bull Epidémiol Hebd. 2017;27–28:586–91. French [cited 2017 Nov 14]. Available from: https://www.santepubliquefrance.fr/maladies-et-traumatismes/diabete/docu....
-
- Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe: baseline results from the Eurodiale study. Diabetologia 2007;50:18–25. 10.1007/s00125-006-0491-1 - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

