Withdrawal of inhaled corticosteroids versus continuation of triple therapy in patients with COPD in real life: observational comparative effectiveness study
- PMID: 33478491
- PMCID: PMC7818945
- DOI: 10.1186/s12931-021-01615-0
Withdrawal of inhaled corticosteroids versus continuation of triple therapy in patients with COPD in real life: observational comparative effectiveness study
Abstract
Background: Inhaled corticosteroids (ICS) are indicated for prevention of exacerbations in patients with COPD, but they are frequently overprescribed. ICS withdrawal has been recommended by international guidelines in order to prevent side effects in patients in whom ICS are not indicated.
Method: Observational comparative effectiveness study aimed to evaluate the effect of ICS withdrawal versus continuation of triple therapy (TT) in COPD patients in primary care. Data were obtained from the Optimum Patient Care Research Database (OPCRD) in the UK.
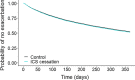
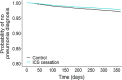
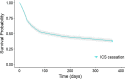
Results: A total of 1046 patients who withdrew ICS were matched 1:4 by time on TT to 4184 patients who continued with TT. Up to 76.1% of the total population had 0 or 1 exacerbation the previous year. After controlling for confounders, patients who discontinued ICS did not have an increased risk of moderate or severe exacerbations (adjusted HR: 1.04, 95% confidence interval (CI) 0.94-1.15; p = 0.441). However, rates of exacerbations managed in primary care (incidence rate ratio (IRR) 1.33, 95% CI 1.10-1.60; p = 0.003) or in hospital (IRR 1.72, 95% CI 1.03-2.86; p = 0.036) were higher in the cessation group. Unsuccessful ICS withdrawal was significantly and independently associated with more frequent courses of oral corticosteroids the previous year and with a blood eosinophil count ≥ 300 cells/μL.
Conclusions: In this primary care population of patients with COPD, composed mostly of infrequent exacerbators, discontinuation of ICS from TT was not associated with an increased risk of exacerbation; however, the subgroup of patients with more frequent courses of oral corticosteroids and high blood eosinophil counts should not be withdrawn from ICS. Trial registration European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (EUPAS30851).
Keywords: COPD; Effectiveness; Inhaled corticosteroids; Real life; Withdrawal.
Conflict of interest statement
Helgo Magnussen reports personal fees from Boehringer Ingelheim during the conduct of the study and personal fees from AstraZeneca, Novartis, and ndd Medical Technologies, Inc. outside the submitted work. Marc Miravitlles has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols and Novartis, consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, Kamada, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, TEVA, Spin Therapeutics, pH Pharma, Novartis, Sanofi and Grifols and research grants from GlaxoSmithKline and Grifols. Sarah Lucas is an employee of the Respiratory Effectiveness Group, who received funding from Boehringer Ingelheim for conducting this study. Jennifer Quint has received grants from The Health Foundation, MRC, GSK, Bayer, BI, British Lung Foundation, IQVIA, Chiesi AZ, Insmed and Asthma UK outside the submitted work; grants and personal fees from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, Bayer, Insmed. Ronald J. Dandurand has received research grants from AstraZeneca Boehringer Ingelheim, GlaxoSmithKline, Novatis, Pfizer and Teva Pharma, consulting fees from Boehringer Ingelheim and Grifols, and speaking fees from Boehringer Ingelheim and Novartis. Nicolas Roche reports grants and personal fees from Boehringer Ingelheim, Novartis, Pfizer and personal fees from Teva, GSK, AstraZeneca, Chiesi, Sanofi, Trudell, Zambon. David Price has board membership with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals, Thermofisher; consultancy agreements with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Novartis, Pfizer, Teva Pharmaceuticals, Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Teva Pharmaceuticals, Theravance, UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma, Novartis; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis, Thermofisher; funding for patient enrolment or completion of research from Novartis; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the Efficacy and Mechanism Evaluation programme, and Health Technology Assessment; and was an expert witness for GlaxoSmithKline.
Figures

Similar articles
-
Blood Eosinophil Counts, Withdrawal of Inhaled Corticosteroids and Risk of COPD Exacerbations and Mortality in the Clinical Practice Research Datalink (CPRD).COPD. 2019 Apr;16(2):152-159. doi: 10.1080/15412555.2019.1608172. Epub 2019 May 23. COPD. 2019. PMID: 31117850
-
Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial.Lancet Respir Med. 2016 May;4(5):390-8. doi: 10.1016/S2213-2600(16)00100-4. Epub 2016 Apr 7. Lancet Respir Med. 2016. PMID: 27066739 Clinical Trial.
-
Comparative effectiveness of LABA-ICS versus LAMA as initial treatment in COPD targeted by blood eosinophils: a population-based cohort study.Lancet Respir Med. 2018 Nov;6(11):855-862. doi: 10.1016/S2213-2600(18)30368-0. Epub 2018 Oct 18. Lancet Respir Med. 2018. PMID: 30343028 Clinical Trial.
-
Withdrawal of Inhaled Corticosteroids from Patients with COPD; Effect on Exacerbation Frequency and Lung Function: A Systematic Review.Int J Chron Obstruct Pulmon Dis. 2024 Jun 21;19:1403-1419. doi: 10.2147/COPD.S436525. eCollection 2024. Int J Chron Obstruct Pulmon Dis. 2024. PMID: 38919905 Free PMC article. Review.
-
Applying the wisdom of stepping down inhaled corticosteroids in patients with COPD: a proposed algorithm for clinical practice.Int J Chron Obstruct Pulmon Dis. 2015 Nov 20;10:2535-48. doi: 10.2147/COPD.S93321. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 26648711 Free PMC article. Review.
Cited by
-
Investigating the rationale for COPD maintenance therapy prescription across Europe, findings from a multi-country study.NPJ Prim Care Respir Med. 2023 May 3;33(1):18. doi: 10.1038/s41533-023-00334-x. NPJ Prim Care Respir Med. 2023. PMID: 37137900 Free PMC article.
-
Pulmonologists' Opinion on the Use of Inhaled Corticosteroids in Chronic Obstructive Pulmonary Disease Patients in Spain: A Cross-Sectional Survey.Int J Chron Obstruct Pulmon Dis. 2022 Jul 12;17:1577-1587. doi: 10.2147/COPD.S369118. eCollection 2022. Int J Chron Obstruct Pulmon Dis. 2022. PMID: 35855745 Free PMC article.
-
Impact of switching from triple therapy to dual bronchodilation in COPD: the DACCORD 'real world' study.Respir Res. 2022 May 2;23(1):109. doi: 10.1186/s12931-022-02037-2. Respir Res. 2022. PMID: 35501806 Free PMC article.
-
Use of single-inhaler triple therapy in the management of obstructive airway disease: Indian medical experts' review.ERJ Open Res. 2022 Mar 28;8(1):00556-2021. doi: 10.1183/23120541.00556-2021. eCollection 2022 Jan. ERJ Open Res. 2022. PMID: 35350278 Free PMC article. Review.
-
A descriptive cohort study of withdrawal from inhaled corticosteroids in COPD patients.NPJ Prim Care Respir Med. 2022 Jul 20;32(1):25. doi: 10.1038/s41533-022-00288-6. NPJ Prim Care Respir Med. 2022. PMID: 35859081 Free PMC article.
References
-
- Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5). - PubMed
-
- Cazzola M, Rogliani P, Calzetta L, Matera MG. Triple therapy versus single and dual long-acting bronchodilator therapy in COPD: a systematic review and meta-analysis. Eur Respir J. 2018; 52(6). - PubMed
-
- Roche N, Anzueto A, Bosnic Anticevich S, Kaplan A, Miravitlles M, Ryan D, et al. Connected real-life research, a pillar of P4 medicine. Eur Respir J. 2020; 55(1). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

