A Novel In Vitro Culture Model System to Study Merkel Cell Polyomavirus-Associated MCC Using Three-Dimensional Organotypic Raft Equivalents of Human Skin
- PMID: 33478104
- PMCID: PMC7835998
- DOI: 10.3390/v13010138
A Novel In Vitro Culture Model System to Study Merkel Cell Polyomavirus-Associated MCC Using Three-Dimensional Organotypic Raft Equivalents of Human Skin
Abstract
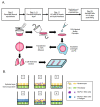
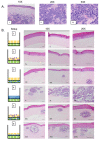
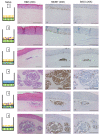
Merkel cell polyomavirus (MCPyV) is a human polyomavirus causally linked to the development of Merkel cell carcinoma (MCC), an aggressive malignancy that largely arises within the dermis of the skin. In this study, we recapitulate the histopathology of human MCC tumors in vitro using an organotypic (raft) culture system that is traditionally used to recapitulate the dermal and epidermal equivalents of skin in three dimensions (3D). In the optimal culture condition, MCPyV+ MCC cells were embedded in collagen between the epidermal equivalent comprising human keratinocytes and a dermal equivalent containing fibroblasts, resulting in MCC-like lesions arising within the dermal equivalent. The presence and organization of MCC cells within these dermal lesions were characterized through biomarker analyses. Interestingly, co-culture of MCPyV+ MCC together with keratinocytes specifically within the epidermal equivalent of the raft did not reproduce human MCC morphology, nor were any keratinocytes necessary for MCC-like lesions to develop in the dermal equivalent. This 3D tissue culture system provides a novel in vitro platform for studying the role of MCPyV T antigens in MCC oncogenesis, identifying additional factors involved in this process, and for screening potential MCPyV+ MCC therapeutic strategies.
Keywords: DNA tumor virus; Merkel cell carcinoma; Merkel cell polyomavirus; Merkel cells; human polyomavirus; organotypic rafts; skin equivalents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Merkel Cell Polyomavirus Downregulates N-myc Downstream-Regulated Gene 1, Leading to Cellular Proliferation and Migration.J Virol. 2020 Jan 17;94(3):e00899-19. doi: 10.1128/JVI.00899-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31694959 Free PMC article.
-
Detection of Merkel cell polyomavirus in Merkel cell carcinomas and small cell carcinomas by PCR and immunohistochemistry.Histol Histopathol. 2011 Oct;26(10):1231-41. doi: 10.14670/HH-26.1231. Histol Histopathol. 2011. PMID: 21870327
-
Merkel cell polyomavirus-specific immune responses in patients with Merkel cell carcinoma receiving anti-PD-1 therapy.J Immunother Cancer. 2018 Nov 27;6(1):131. doi: 10.1186/s40425-018-0450-7. J Immunother Cancer. 2018. PMID: 30482247 Free PMC article.
-
Are there multiple cells of origin of Merkel cell carcinoma?Oncogene. 2018 Mar;37(11):1409-1416. doi: 10.1038/s41388-017-0073-3. Epub 2018 Jan 11. Oncogene. 2018. PMID: 29321666 Free PMC article. Review.
-
Merkel cell polyomavirus and Merkel cell carcinoma.Philos Trans R Soc Lond B Biol Sci. 2017 Oct 19;372(1732):20160276. doi: 10.1098/rstb.2016.0276. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28893943 Free PMC article. Review.
Cited by
-
Novel methodologies for host-microbe interactions and microbiome-targeted therapeutics in 3D organotypic skin models.Microbiome. 2023 Oct 17;11(1):227. doi: 10.1186/s40168-023-01668-x. Microbiome. 2023. PMID: 37849006 Free PMC article.
-
Interactions between avian viruses and skin in farm birds.Vet Res. 2024 Apr 26;55(1):54. doi: 10.1186/s13567-024-01310-0. Vet Res. 2024. PMID: 38671518 Free PMC article. Review.
-
Current In Vitro and In Vivo Models to Study MCPyV-Associated MCC.Viruses. 2022 Oct 7;14(10):2204. doi: 10.3390/v14102204. Viruses. 2022. PMID: 36298759 Free PMC article. Review.
-
Organotypic Epithelial Raft Cultures as a Three-Dimensional In Vitro Model of Merkel Cell Carcinoma.Cancers (Basel). 2022 Feb 21;14(4):1091. doi: 10.3390/cancers14041091. Cancers (Basel). 2022. PMID: 35205840 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

