The Effect of Hypoxia on the Expression of CXC Chemokines and CXC Chemokine Receptors-A Review of Literature
- PMID: 33467722
- PMCID: PMC7830156
- DOI: 10.3390/ijms22020843
The Effect of Hypoxia on the Expression of CXC Chemokines and CXC Chemokine Receptors-A Review of Literature
Abstract
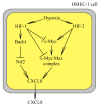
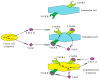
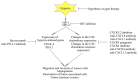
Hypoxia is an integral component of the tumor microenvironment. Either as chronic or cycling hypoxia, it exerts a similar effect on cancer processes by activating hypoxia-inducible factor-1 (HIF-1) and nuclear factor (NF-κB), with cycling hypoxia showing a stronger proinflammatory influence. One of the systems affected by hypoxia is the CXC chemokine system. This paper reviews all available information on hypoxia-induced changes in the expression of all CXC chemokines (CXCL1, CXCL2, CXCL3, CXCL4, CXCL5, CXCL6, CXCL7, CXCL8 (IL-8), CXCL9, CXCL10, CXCL11, CXCL12 (SDF-1), CXCL13, CXCL14, CXCL15, CXCL16, CXCL17) as well as CXC chemokine receptors-CXCR1, CXCR2, CXCR3, CXCR4, CXCR5, CXCR6, CXCR7 and CXCR8. First, we present basic information on the effect of these chemoattractant cytokines on cancer processes. We then discuss the effect of hypoxia-induced changes on CXC chemokine expression on the angiogenesis, lymphangiogenesis and recruitment of various cells to the tumor niche, including myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), regulatory T cells (Tregs) and tumor-infiltrating lymphocytes (TILs). Finally, the review summarizes data on the use of drugs targeting the CXC chemokine system in cancer therapies.
Keywords: CXC chemokine; HIF-1α; IL-8; NF-κB; SDF-1; cancer; cycling hypoxia; hypoxia; hypoxia-inducible factor; tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Role of CXC group chemokines in lung cancer development and progression.J Thorac Dis. 2017 Apr;9(Suppl 3):S164-S171. doi: 10.21037/jtd.2017.03.61. J Thorac Dis. 2017. PMID: 28446981 Free PMC article.
-
Exploration of prognostic biomarkers and therapeutic targets in the microenvironment of bladder cancer based on CXC chemokines.Math Biosci Eng. 2021 Jul 19;18(5):6262-6287. doi: 10.3934/mbe.2021313. Math Biosci Eng. 2021. PMID: 34517533
-
Chemokine-cytokine cross-talk. The ELR+ CXC chemokine LIX (CXCL5) amplifies a proinflammatory cytokine response via a phosphatidylinositol 3-kinase-NF-kappa B pathway.J Biol Chem. 2003 Feb 14;278(7):4675-86. doi: 10.1074/jbc.M207006200. Epub 2002 Dec 4. J Biol Chem. 2003. PMID: 12468547
-
The role of CXC chemokines and their receptors in cancer.Cancer Lett. 2008 Aug 28;267(2):226-44. doi: 10.1016/j.canlet.2008.04.050. Epub 2008 Jun 24. Cancer Lett. 2008. PMID: 18579287 Review.
-
Advance in the role of chemokines/chemokine receptors in carcinogenesis: Focus on pancreatic cancer.Eur J Pharmacol. 2024 Mar 15;967:176357. doi: 10.1016/j.ejphar.2024.176357. Epub 2024 Feb 1. Eur J Pharmacol. 2024. PMID: 38309677 Review.
Cited by
-
Regulatory Networks, Management Approaches, and Emerging Treatments of Nonalcoholic Fatty Liver Disease.Can J Gastroenterol Hepatol. 2022 Nov 8;2022:6799414. doi: 10.1155/2022/6799414. eCollection 2022. Can J Gastroenterol Hepatol. 2022. PMID: 36397950 Free PMC article. Review.
-
Interleukin-35 exhibits protective effects in a rat model of hypoxic-ischemic encephalopathy through the inhibition of microglia-mediated inflammation.Transl Pediatr. 2022 May;11(5):651-662. doi: 10.21037/tp-22-100. Transl Pediatr. 2022. PMID: 35685068 Free PMC article.
-
The Polygenic Map of Keloid Fibroblasts Reveals Fibrosis-Associated Gene Alterations in Inflammation and Immune Responses.Front Immunol. 2022 Jan 10;12:810290. doi: 10.3389/fimmu.2021.810290. eCollection 2021. Front Immunol. 2022. PMID: 35082796 Free PMC article.
-
Efficacy of Cisplatin-CXCR4 Antagonist Combination Therapy in Oral Cancer.Cancers (Basel). 2024 Jun 25;16(13):2326. doi: 10.3390/cancers16132326. Cancers (Basel). 2024. PMID: 39001388 Free PMC article.
-
Exhaust the exhausters: Targeting regulatory T cells in the tumor microenvironment.Front Immunol. 2022 Sep 30;13:940052. doi: 10.3389/fimmu.2022.940052. eCollection 2022. Front Immunol. 2022. PMID: 36248808 Free PMC article. Review.
References
-
- GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. - DOI - PMC - PubMed
-
- Mu X., Shi W., Xu Y., Xu C., Zhao T., Geng B., Yang J., Pan J., Hu S., Zhang C., et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle. 2018;17:428–438. doi: 10.1080/15384101.2018.1444305. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

