The Cycling of Intracellular Calcium Released in Response to Fluid Shear Stress Is Critical for Migration-Associated Actin Reorganization in Eosinophils
- PMID: 33467432
- PMCID: PMC7829934
- DOI: 10.3390/cells10010157
The Cycling of Intracellular Calcium Released in Response to Fluid Shear Stress Is Critical for Migration-Associated Actin Reorganization in Eosinophils
Abstract
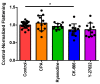
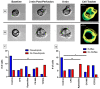
The magnitude of eosinophil mobilization into respiratory tissues drives the severity of inflammation in several airway diseases. In classical models of leukocyte extravasation, surface integrins undergo conformational switches to high-affinity states via chemokine binding activation. Recently, we learned that eosinophil integrins possess mechanosensitive properties that detect fluid shear stress, which alone was sufficient to induce activation. This mechanical stimulus triggered intracellular calcium release and hallmark migration-associated cytoskeletal reorganization including flattening for increased cell-substratum contact area and pseudopodia formation. The present study utilized confocal fluorescence microscopy to investigate the effects of pharmacological inhibitors to calcium signaling and actin polymerization pathways on shear stress-induced migration in vitro. Morphological changes (cell elongation, membrane protrusions) succeeded the calcium flux in untreated eosinophils within 2 min, suggesting that calcium signaling was upstream of actin cytoskeleton rearrangement. The inhibition of ryanodine receptors and endomembrane Ca2+-ATPases corroborated this idea, indicated by a significant increase in time between the calcium spike and actin polymerization. The impact of the temporal link is evident as the capacity of treated eosinophils to move across fibronectin-coated surfaces was significantly hampered relative to untreated eosinophils. Furthermore, we determined that the nature of cellular motility in response to fluid shear stress was nondirectional.
Keywords: actin; calcium signaling; confocal microscopy; integrin; mechanosensing; membrane ruffles; pseudopodia; shear stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
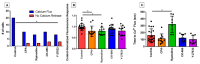
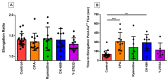
Similar articles
-
The eosinophil actin cytoskeleton undergoes rapid rearrangement in response to fluid shear stress.J Leukoc Biol. 2020 Jul;108(1):129-137. doi: 10.1002/JLB.1MA0320-349RR. Epub 2020 Apr 29. J Leukoc Biol. 2020. PMID: 32349177
-
Requirement of Ca2+ influx- and phosphatidylinositol 3-kinase-mediated m-calpain activity for shear stress-induced endothelial cell polarity.Am J Physiol Cell Physiol. 2007 Oct;293(4):C1216-25. doi: 10.1152/ajpcell.00083.2007. Epub 2007 Jun 27. Am J Physiol Cell Physiol. 2007. PMID: 17596297
-
Ca(2+) regulates fluid shear-induced cytoskeletal reorganization and gene expression in osteoblasts.Am J Physiol Cell Physiol. 2000 May;278(5):C989-97. doi: 10.1152/ajpcell.2000.278.5.C989. Am J Physiol Cell Physiol. 2000. PMID: 10794673
-
Membrane Ruffles: Composition, Function, Formation and Visualization.Int J Mol Sci. 2024 Oct 12;25(20):10971. doi: 10.3390/ijms252010971. Int J Mol Sci. 2024. PMID: 39456754 Free PMC article. Review.
-
Response of cells and tissues to shear stress.J Cell Sci. 2023 Sep 15;136(18):jcs260985. doi: 10.1242/jcs.260985. Epub 2023 Sep 25. J Cell Sci. 2023. PMID: 37747423 Free PMC article. Review.
Cited by
-
Eosinophils, beyond IL-5.Cells. 2021 Oct 1;10(10):2615. doi: 10.3390/cells10102615. Cells. 2021. PMID: 34685594 Free PMC article.
-
MoCbp7, a Novel Calcineurin B Subunit-Binding Protein, Is Involved in the Calcium Signaling Pathway and Regulates Fungal Development, Virulence, and ER Homeostasis in Magnaporthe oryzae.Int J Mol Sci. 2023 May 26;24(11):9297. doi: 10.3390/ijms24119297. Int J Mol Sci. 2023. PMID: 37298247 Free PMC article.
-
Roles of mechanosensitive ion channels in immune cells.Heliyon. 2023 Dec 4;10(1):e23318. doi: 10.1016/j.heliyon.2023.e23318. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38148826 Free PMC article. Review.
-
A new side-effect of sufentanil: increased monocyte-endothelial adhesion.BMC Anesthesiol. 2021 Nov 3;21(1):267. doi: 10.1186/s12871-021-01487-3. BMC Anesthesiol. 2021. PMID: 34732147 Free PMC article.
-
Ca2+-Dependent Processes of Innate Immunity in IBD.Cells. 2024 Jun 21;13(13):1079. doi: 10.3390/cells13131079. Cells. 2024. PMID: 38994933 Free PMC article. Review.
References
-
- Lima-Matos A., Ponte E.V., de Jesus J.P.V., Almeida P.C.A., Lima V.B., Kwon N., Riley J., de Mello L.M., Cruz A.A. Eosinophilic asthma, according to a blood eosinophil criterion, is associated with disease severity and lack of control among underprivileged urban Brazilians. Respir. Med. 2018;145:95–100. doi: 10.1016/j.rmed.2018.10.025. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

