Histamine H1 receptor antagonists selectively kill cisplatin-resistant human cancer cells
- PMID: 33452347
- PMCID: PMC7810706
- DOI: 10.1038/s41598-021-81077-y
Histamine H1 receptor antagonists selectively kill cisplatin-resistant human cancer cells
Abstract
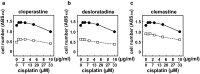
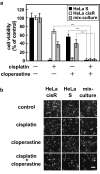
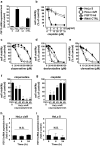
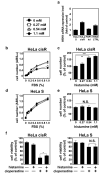
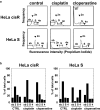
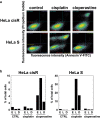
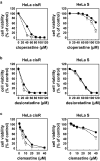
Cancer therapy is often hampered by the disease's development of resistance to anticancer drugs. We previously showed that the autonomously upregulated product of fibroblast growth factor 13 gene (FGF13; also known as FGF homologous factor 2 (FHF2)) is responsible for the cisplatin resistance of HeLa cisR cells and that it is likely responsible for the poor prognosis of cervical cancer patients treated with cisplatin. Here we show that cloperastine and two other histamine H1 receptor antagonists selectively kill HeLa cisR cells at concentrations that little affect parental HeLa S cells. The sensitivity of HeLa cisR cells to cloperastine was abolished by knocking down FGF13 expression. Cisplatin-resistant A549 cisR cells were similarly susceptible to cloperastine. H2, H3, and H4 receptor antagonists showed less or no cytotoxicity toward HeLa cisR or A549 cisR cells. These results indicate that histamine H1 receptor antagonists selectively kill cisplatin-resistant human cancer cells and suggest that this effect is exerted through a molecular mechanism involving autocrine histamine activity and high-level expression of FGF13. We think this represents a potential opportunity to utilize H1 receptor antagonists in combination with anticancer agents to treat cancers in which emergent drug-resistance is preventing effective treatment.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Upregulated expression of FGF13/FHF2 mediates resistance to platinum drugs in cervical cancer cells.Sci Rep. 2013 Oct 11;3:2899. doi: 10.1038/srep02899. Sci Rep. 2013. PMID: 24113164 Free PMC article.
-
Histamine H1-receptor antagonists inhibit nuclear factor-kappaB and activator protein-1 activities via H1-receptor-dependent and -independent mechanisms.Clin Exp Allergy. 2008 Jun;38(6):947-56. doi: 10.1111/j.1365-2222.2008.02990.x. Clin Exp Allergy. 2008. PMID: 18498541
-
Characterization of the histamine receptors in the guinea-pig lung: evidence for relaxant histamine H3 receptors in the trachea.Br J Pharmacol. 1994 Feb;111(2):445-54. doi: 10.1111/j.1476-5381.1994.tb14756.x. Br J Pharmacol. 1994. PMID: 7911715 Free PMC article.
-
Characterization and distribution of histamine H1- and H2-receptors in precapillary vessels.J Cardiovasc Pharmacol. 1984;6 Suppl 4:S587-97. doi: 10.1097/00005344-198406004-00005. J Cardiovasc Pharmacol. 1984. PMID: 6083401 Review.
-
Perspectives in Drug Development and Clinical Pharmacology: The Discovery of Histamine H1 and H2 Antagonists.Clin Pharmacol Drug Dev. 2016 Jan;5(1):5-12. doi: 10.1002/cpdd.236. Clin Pharmacol Drug Dev. 2016. PMID: 27119574 Review.
Cited by
-
Expression profile of messenger and micro RNAs related to the histaminergic system in patients with five subtypes of breast cancer.Front Oncol. 2024 Aug 29;14:1407538. doi: 10.3389/fonc.2024.1407538. eCollection 2024. Front Oncol. 2024. PMID: 39267843 Free PMC article.
-
Antihistamines H1 use on survival outcomes in esophageal squamous cell carcinoma patients undergoing concurrent chemoradiotherapy.Am J Cancer Res. 2023 Nov 15;13(11):5733-5745. eCollection 2023. Am J Cancer Res. 2023. PMID: 38058841 Free PMC article.
-
Association of Antihistamine Use with Increased Risk of Esophageal Squamous Cell Carcinoma: A Nationwide, Long-Term Follow-Up Study Using Propensity Score Matching.Biomedicines. 2023 Feb 16;11(2):578. doi: 10.3390/biomedicines11020578. Biomedicines. 2023. PMID: 36831114 Free PMC article.
-
The Multidirectional Effect of Azelastine Hydrochloride on Cervical Cancer Cells.Int J Mol Sci. 2022 May 24;23(11):5890. doi: 10.3390/ijms23115890. Int J Mol Sci. 2022. PMID: 35682572 Free PMC article.
-
Role of curcumin in ischemia and reperfusion injury.Front Pharmacol. 2023 Mar 20;14:1057144. doi: 10.3389/fphar.2023.1057144. eCollection 2023. Front Pharmacol. 2023. PMID: 37021057 Free PMC article. Review.
References
-
- Okada T, Murata K, Hirose R, Matsuda C, Komatsu T, Ikekita M, Nakawatari M, Nakayama F, Wakatsuki M, Ohno T, Kato S, Imai T, Imamura T. Upregulated expression of FGF13/FHF2 mediates resistance to platinum drugs in cervical cancer cells. Sci. Rep. 2013;3:2899. doi: 10.1038/srep02899. - DOI - PMC - PubMed
-
- Bublik DR, Bursać S, Sheffer M, Oršolić I, Shalit T, Tarcic O, Kotler E, Mouhadeb O, Hoffman Y, Fuchs G, Levin Y, Volarević S, Oren M. Regulatory module involving FGF13, miR-504, and p53 regulates ribosomal biogenesis and supports cancer cell survival. Proc. Natl. Acad. Sci. U.S.A. 2017;114:E496–E505. doi: 10.1073/pnas.1614876114. - DOI - PMC - PubMed
-
- Jayasimha RD, Basha S, Kotha P, Katike U. Designing, docking and molecular dynamics simulation studies of novel cloperastine analogues as anti-allergic agents: Homology modeling and active site prediction for the human histamine H1 receptor. RSC Adv. 2020;10:4745–4754. doi: 10.1039/C9RA09245E. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

