A practical guide to preclinical systematic review and meta-analysis
- PMID: 33449500
- PMCID: PMC7431149
- DOI: 10.1097/j.pain.0000000000001974
A practical guide to preclinical systematic review and meta-analysis
Conflict of interest statement
The authors have no conflicts of interest to declare.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures

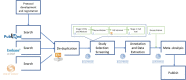
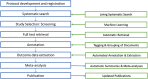
Similar articles
-
Comparison of freshly cultured versus freshly thawed (cryopreserved) mesenchymal stem cells in preclinical in vivo models of inflammation: a protocol for a preclinical systematic review and meta-analysis.Syst Rev. 2020 Aug 19;9(1):188. doi: 10.1186/s13643-020-01437-z. Syst Rev. 2020. PMID: 32814560 Free PMC article.
-
A practical guide to understanding systematic reviews and meta-analyses.Otolaryngol Head Neck Surg. 2010 Jan;142(1):6-14. doi: 10.1016/j.otohns.2009.09.005. Epub 2009 Nov 26. Otolaryngol Head Neck Surg. 2010. PMID: 20096216
-
Meta-analysis of data from animal studies: a practical guide.J Neurosci Methods. 2014 Jan 15;221:92-102. doi: 10.1016/j.jneumeth.2013.09.010. Epub 2013 Oct 4. J Neurosci Methods. 2014. PMID: 24099992
-
A practical guide to systematic literature reviews and meta-analyses in infection prevention: Planning, challenges, and execution.Am J Infect Control. 2017 Nov 1;45(11):1292-1294. doi: 10.1016/j.ajic.2017.08.004. Epub 2017 Sep 13. Am J Infect Control. 2017. PMID: 28918302 Review.
-
A methodological review with meta-epidemiological analysis of preclinical systematic reviews with meta-analyses.Sci Rep. 2022 Nov 21;12(1):20066. doi: 10.1038/s41598-022-24447-4. Sci Rep. 2022. PMID: 36414712 Free PMC article. Review.
Cited by
-
The power of integrating data: advancing pain research using meta-analysis.Pain Rep. 2022 Oct 4;7(6):e1038. doi: 10.1097/PR9.0000000000001038. eCollection 2022 Nov-Dec. Pain Rep. 2022. PMID: 36213594 Free PMC article. Review.
-
Value of preclinical systematic reviews and meta-analyses in pediatric research.Pediatr Res. 2024 Aug;96(3):643-653. doi: 10.1038/s41390-024-03197-1. Epub 2024 Apr 13. Pediatr Res. 2024. PMID: 38615075 Free PMC article.
-
A meta-analysis of animal studies evaluating the effect of hydrogen sulfide on ischemic stroke: is the preclinical evidence sufficient to move forward?Naunyn Schmiedebergs Arch Pharmacol. 2024 Dec;397(12):9533-9548. doi: 10.1007/s00210-024-03291-5. Epub 2024 Jul 17. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 39017715 Free PMC article. Review.
-
Genetically modified animals as models of neurodevelopmental conditions: A review of systematic review reporting quality.Brain Neurosci Adv. 2024 Oct 18;8:23982128241287279. doi: 10.1177/23982128241287279. eCollection 2024 Jan-Dec. Brain Neurosci Adv. 2024. PMID: 39431203 Free PMC article.
-
Fibroblasts: the neglected cell type in peripheral sensitisation and chronic pain? A review based on a systematic search of the literature.BMJ Open Sci. 2022 Jan 18;6(1):e100235. doi: 10.1136/bmjos-2021-100235. eCollection 2022. BMJ Open Sci. 2022. PMID: 35128075 Free PMC article.
References
-
- Andrews NA, Latrémolière A, Basbaum AI, Mogil JS, Porreca F, Rice ASC, Woolf CJ, Currie GL, Dworkin RH, Eisenach JC, Evans S, Gewandter JS, Gover TD, Handwerker H, Huang W, Iyengar S, Jensen MP, Kennedy JD, Lee N, Levine J, Lidster K, Machin I, McDermott MP, McMahon SB, Price TJ, Ross SE, Scherrer G, Seal RP, Sena ES, Silva E, Stone L, Svensson CI, Turk DC, Whiteside G. Ensuring transparency and minimization of methodologic bias in preclinical pain research: PPRECISE considerations. PAIN 2016;157:901–9. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

