New Nanoparticle Formulation for Cyclosporin A: In Vitro Assessment
- PMID: 33445646
- PMCID: PMC7828155
- DOI: 10.3390/pharmaceutics13010091
New Nanoparticle Formulation for Cyclosporin A: In Vitro Assessment
Abstract
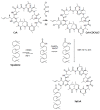
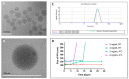
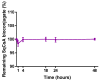
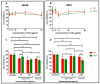
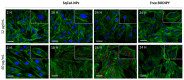
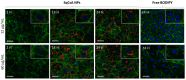
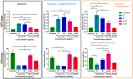
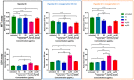
Cyclosporin A (CsA) is a molecule with well-known immunosuppressive properties. As it also acts on the opening of mitochondrial permeability transition pore (mPTP), CsA has been evaluated for ischemic heart diseases (IHD). However, its distribution throughout the body and its physicochemical characteristics strongly limit the use of CsA for intravenous administration. In this context, nanoparticles (NPs) have emerged as an opportunity to circumvent the above-mentioned limitations. We have developed in our laboratory an innovative nanoformulation based on the covalent bond between squalene (Sq) and cyclosporin A to avoid burst release phenomena and increase drug loading. After a thorough characterization of the bioconjugate, we proceeded with a nanoprecipitation in aqueous medium in order to obtain SqCsA NPs of well-defined size. The SqCsA NPs were further characterized using dynamic light scattering (DLS), cryogenic transmission electron microscopy (cryoTEM), and high-performance liquid chromatography (HPLC), and their cytotoxicity was evaluated. As the goal is to employ them for IHD, we evaluated the cardioprotective capacity on two cardiac cell lines. A strong cardioprotective effect was observed on cardiomyoblasts subjected to experimental hypoxia/reoxygenation. Further research is needed in order to understand the mechanisms of action of SqCsA NPs in cells. This new formulation of CsA could pave the way for possible medical application.
Keywords: bioconjugate; cardiac cell line; cellular uptake; cyclosporin A; cytotoxicity; squalene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Development and Characterization of Innovative Multidrug Nanoformulation for Cardiac Therapy.Materials (Basel). 2023 Feb 22;16(5):1812. doi: 10.3390/ma16051812. Materials (Basel). 2023. PMID: 36902927 Free PMC article.
-
Mitochondria-targeted cyclosporin A delivery system to treat myocardial ischemia reperfusion injury of rats.J Nanobiotechnology. 2019 Jan 25;17(1):18. doi: 10.1186/s12951-019-0451-9. J Nanobiotechnology. 2019. PMID: 30683110 Free PMC article.
-
Tissue kallikrein is required for the cardioprotective effect of cyclosporin A in myocardial ischemia in the mouse.Biochem Pharmacol. 2015 Mar 1;94(1):22-9. doi: 10.1016/j.bcp.2015.01.007. Epub 2015 Jan 23. Biochem Pharmacol. 2015. PMID: 25623731
-
Mitochondrial permeability transition pore as a target for cardioprotection in the human heart.Am J Physiol Heart Circ Physiol. 2005 Jul;289(1):H237-42. doi: 10.1152/ajpheart.01192.2004. Am J Physiol Heart Circ Physiol. 2005. PMID: 15961375
-
Effects of silencing the RET/PTC1 oncogene in papillary thyroid carcinoma by siRNA-squalene nanoparticles with and without fusogenic companion GALA-cholesterol.Thyroid. 2014 Feb;24(2):327-38. doi: 10.1089/thy.2012.0544. Epub 2014 Jan 9. Thyroid. 2014. PMID: 23885719
Cited by
-
Assessment of Squalene-Adenosine Nanoparticles in Two Rodent Models of Cardiac Ischemia-Reperfusion.Pharmaceutics. 2023 Jun 21;15(7):1790. doi: 10.3390/pharmaceutics15071790. Pharmaceutics. 2023. PMID: 37513977 Free PMC article.
-
Therapeutic Peptides to Treat Myocardial Ischemia-Reperfusion Injury.Front Cardiovasc Med. 2022 Feb 17;9:792885. doi: 10.3389/fcvm.2022.792885. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35252383 Free PMC article. Review.
-
Development and Characterization of Innovative Multidrug Nanoformulation for Cardiac Therapy.Materials (Basel). 2023 Feb 22;16(5):1812. doi: 10.3390/ma16051812. Materials (Basel). 2023. PMID: 36902927 Free PMC article.
-
Bio-synthesis, purification and structural analysis of Cyclosporine-A produced by Tolypocladium inflatum with valorization of agro-industrial wastes.Sci Rep. 2024 May 31;14(1):12540. doi: 10.1038/s41598-024-63110-y. Sci Rep. 2024. PMID: 38822034 Free PMC article.
-
Current Updates on Potential Role of Flavonoids in Hypoxia/Reoxygenation Cardiac Injury Model.Cardiovasc Toxicol. 2021 Aug;21(8):605-618. doi: 10.1007/s12012-021-09666-x. Epub 2021 Jun 10. Cardiovasc Toxicol. 2021. PMID: 34114196 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

