Norovirus-Specific CD8+ T Cell Responses in Human Blood and Tissues
- PMID: 33444817
- PMCID: PMC8010716
- DOI: 10.1016/j.jcmgh.2020.12.012
Norovirus-Specific CD8+ T Cell Responses in Human Blood and Tissues
Abstract
Background & aims: Noroviruses (NoVs) are the leading cause of acute gastroenteritis worldwide and are associated with significant morbidity and mortality. Moreover, an asymptomatic carrier state can persist following acute infection, promoting NoV spread and evolution. Thus, defining immune correlates of NoV protection and persistence is needed to guide the development of future vaccines and limit viral spread. Whereas antibody responses following NoV infection or vaccination have been studied extensively, cellular immunity has received less attention. Data from the mouse NoV model suggest that T cells are critical for preventing persistence and achieving viral clearance, but little is known about NoV-specific T-cell immunity in humans, particularly at mucosal sites.
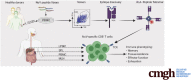
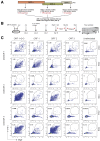
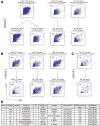
Methods: We screened peripheral blood mononuclear cells from 3 volunteers with an overlapping NoV peptide library. We then used HLA-peptide tetramers to track virus-specific CD8+ T cells in peripheral, lymphoid, and intestinal tissues. Tetramer+ cells were further characterized using markers for cellular trafficking, exhaustion, cytotoxicity, and proliferation.
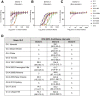
Results: We defined 7 HLA-restricted immunodominant class I epitopes that were highly conserved across pandemic strains from genogroup II.4. NoV-specific CD8+ T cells with central, effector, or tissue-resident memory phenotypes were present at all sites and were especially abundant in the intestinal lamina propria. The properties and differentiation states of tetramer+ cells varied across donors and epitopes.
Conclusions: Our findings are an important step toward defining the breadth, distribution, and properties of human NoV T-cell immunity. Moreover, the molecular tools we have developed can be used to evaluate future vaccines and engineer novel cellular therapeutics.
Keywords: Norovirus T(RM); Norovirus Tetramers; Norovirus-Specific T Cells; T Cell Epitopes.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




Comment in
-
Crossing the T's on Norovirus.Cell Mol Gastroenterol Hepatol. 2021;11(5):1543-1544. doi: 10.1016/j.jcmgh.2021.01.021. Epub 2021 Feb 19. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33617792 Free PMC article. No abstract available.
Similar articles
-
Identification of a First Human Norovirus CD8+ T Cell Epitope Restricted to HLA-A*0201 Allele.Front Immunol. 2018 Nov 27;9:2782. doi: 10.3389/fimmu.2018.02782. eCollection 2018. Front Immunol. 2018. PMID: 30542352 Free PMC article.
-
Persistent enteric murine norovirus infection is associated with functionally suboptimal virus-specific CD8 T cell responses.J Virol. 2013 Jun;87(12):7015-31. doi: 10.1128/JVI.03389-12. Epub 2013 Apr 17. J Virol. 2013. PMID: 23596300 Free PMC article.
-
Broad blockade antibody responses in human volunteers after immunization with a multivalent norovirus VLP candidate vaccine: immunological analyses from a phase I clinical trial.PLoS Med. 2015 Mar 24;12(3):e1001807. doi: 10.1371/journal.pmed.1001807. eCollection 2015 Mar. PLoS Med. 2015. PMID: 25803642 Free PMC article. Clinical Trial.
-
Norovirus immunology: Of mice and mechanisms.Eur J Immunol. 2015 Oct;45(10):2742-57. doi: 10.1002/eji.201545512. Epub 2015 Aug 25. Eur J Immunol. 2015. PMID: 26256101 Free PMC article. Review.
-
Norovirus vaccines: Correlates of protection, challenges and limitations.Hum Vaccin Immunother. 2016 Jul 2;12(7):1653-69. doi: 10.1080/21645515.2015.1125054. Epub 2016 Feb 2. Hum Vaccin Immunother. 2016. PMID: 26836766 Free PMC article. Review.
Cited by
-
Inhibition of Norovirus GII.4 binding to HBGAs by Sargassum fusiforme polysaccharide.Biosci Rep. 2024 Sep 25;44(9):BSR20240092. doi: 10.1042/BSR20240092. Biosci Rep. 2024. PMID: 39158037 Free PMC article.
-
A quadrivalent norovirus vaccine based on a chimpanzee adenovirus vector induces potent immunity in mice.Virol Sin. 2024 Aug;39(4):675-684. doi: 10.1016/j.virs.2024.07.002. Epub 2024 Jul 10. Virol Sin. 2024. PMID: 38997087 Free PMC article.
-
Norovirus: Facts and Reflections from Past, Present, and Future.Viruses. 2021 Nov 30;13(12):2399. doi: 10.3390/v13122399. Viruses. 2021. PMID: 34960668 Free PMC article. Review.
-
Noroviruses: Evolutionary Dynamics, Epidemiology, Pathogenesis, and Vaccine Advances-A Comprehensive Review.Vaccines (Basel). 2024 May 29;12(6):590. doi: 10.3390/vaccines12060590. Vaccines (Basel). 2024. PMID: 38932319 Free PMC article. Review.
-
Simvastatin Reduces Protection and Intestinal T Cell Responses Induced by a Norovirus P Particle Vaccine in Gnotobiotic Pigs.Pathogens. 2021 Jul 1;10(7):829. doi: 10.3390/pathogens10070829. Pathogens. 2021. PMID: 34357979 Free PMC article.
References
-
- Tomov V.T., Palko O., Lau C.W., Pattekar A., Sun Y., Tacheva R., Bengsch B., Manne S., Cosma G.L., Eisenlohr L.C., Nice T.J., Virgin H.W., Wherry E.J. Differentiation and protective capacity of virus-specific CD8(+) T cells suggest murine norovirus persistence in an immune-privileged enteric niche. Immunity. 2017;47:723–738 e5. - PMC - PubMed
-
- Payne D.C., Vinje J., Szilagyi P.G., Edwards K.M., Staat M.A., Weinberg G.A., Hall C.B., Chappell J., Bernstein D.I., Curns A.T., Wikswo M., Shirley S.H., Hall A.J., Lopman B., Parashar U.D. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med. 2013;368:1121–1130. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

