Identification and Characterization of Cancer Cells That Initiate Metastases to the Brain and Other Organs
- PMID: 33443114
- PMCID: PMC9281611
- DOI: 10.1158/1541-7786.MCR-20-0863
Identification and Characterization of Cancer Cells That Initiate Metastases to the Brain and Other Organs
Abstract
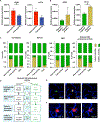
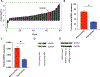
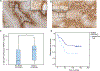
Specific biological properties of those circulating cancer cells that are the origin of brain metastases (BM) are not well understood. Here, single circulating breast cancer cells were fate-tracked during all steps of the brain metastatic cascade in mice after intracardial injection over weeks. A novel in vivo two-photon microscopy methodology was developed that allowed to determine the specific cellular and molecular features of breast cancer cells that homed in the brain, extravasated, and successfully established a brain macrometastasis. Those BM-initiating breast cancer cells (BMIC) were mainly originating from a slow-cycling subpopulation that included only 16% to 20% of all circulating cancer cells. BMICs showed enrichment of various markers of cellular stemness. As a proof of principle for the principal usefulness of this approach, expression profiling of BMICs versus non-BMICs was performed, which revealed upregulation of NDRG1 in the slow-cycling BMIC subpopulation in one BM model. Here, BM development was completely suppressed when NDRG1 expression was downregulated. In accordance, in primary human breast cancer, NDRG1 expression was heterogeneous, and high NDRG1 expression was associated with shorter metastasis-free survival. In conclusion, our data identify temporary slow-cycling breast cancer cells as the dominant source of brain and other metastases and demonstrates that this can lead to better understanding of BMIC-relevant pathways, including potential new approaches to prevent BM in patients. IMPLICATIONS: Cancer cells responsible for successful brain metastasis outgrowth are slow cycling and harbor stemness features. The molecular characteristics of these metastasis-initiating cells can be studied using intravital microscopy technology.
©2020 American Association for Cancer Research.
Figures



Similar articles
-
RNAi screen identifies essential regulators of human brain metastasis-initiating cells.Acta Neuropathol. 2017 Dec;134(6):923-940. doi: 10.1007/s00401-017-1757-z. Epub 2017 Aug 1. Acta Neuropathol. 2017. PMID: 28766011
-
STAT3 pathway regulates lung-derived brain metastasis initiating cell capacity through miR-21 activation.Oncotarget. 2015 Sep 29;6(29):27461-77. doi: 10.18632/oncotarget.4742. Oncotarget. 2015. PMID: 26314961 Free PMC article.
-
Brain metastasis-initiating cells: survival of the fittest.Int J Mol Sci. 2014 May 22;15(5):9117-33. doi: 10.3390/ijms15059117. Int J Mol Sci. 2014. PMID: 24857921 Free PMC article. Review.
-
Therapeutic Targeting of the Premetastatic Stage in Human Lung-to-Brain Metastasis.Cancer Res. 2018 Sep 1;78(17):5124-5134. doi: 10.1158/0008-5472.CAN-18-1022. Epub 2018 Jul 9. Cancer Res. 2018. PMID: 29986997
-
Brain malignancies: Glioblastoma and brain metastases.Semin Cancer Biol. 2020 Feb;60:262-273. doi: 10.1016/j.semcancer.2019.10.010. Epub 2019 Oct 22. Semin Cancer Biol. 2020. PMID: 31654711 Review.
Cited by
-
Monitoring Cell Proliferation by Dye Dilution: Considerations for Panel Design.Methods Mol Biol. 2024;2779:159-216. doi: 10.1007/978-1-0716-3738-8_9. Methods Mol Biol. 2024. PMID: 38526787 Review.
-
Prediction of Short and Long Survival after Surgery for Breast Cancer Brain Metastases.Cancers (Basel). 2022 Mar 10;14(6):1437. doi: 10.3390/cancers14061437. Cancers (Basel). 2022. PMID: 35326590 Free PMC article.
-
NDRG1 in Aggressive Breast Cancer Progression and Brain Metastasis.J Natl Cancer Inst. 2022 Apr 11;114(4):579-591. doi: 10.1093/jnci/djab222. J Natl Cancer Inst. 2022. PMID: 34893874 Free PMC article.
-
Animal models of brain metastasis.Neurooncol Adv. 2021 Nov 27;3(Suppl 5):v144-v156. doi: 10.1093/noajnl/vdab115. eCollection 2021 Nov. Neurooncol Adv. 2021. PMID: 34859241 Free PMC article.
-
Active Remodeling of Capillary Endothelium via Cancer Cell-Derived MMP9 Promotes Metastatic Brain Colonization.Cancer Res. 2023 Apr 14;83(8):1299-1314. doi: 10.1158/0008-5472.CAN-22-3964. Cancer Res. 2023. PMID: 36652557 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

