Reducing Spread of Infections with a Photocatalytic Reactor-Potential Applications in Control of Hospital Staphylococcus aureus and Clostridioides difficile Infections and Inactivation of RNA Viruses
- PMID: 33440699
- PMCID: PMC7838977
- DOI: 10.3390/idr13010008
Reducing Spread of Infections with a Photocatalytic Reactor-Potential Applications in Control of Hospital Staphylococcus aureus and Clostridioides difficile Infections and Inactivation of RNA Viruses
Abstract
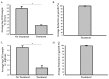
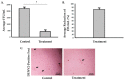
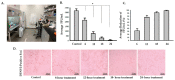
Contaminated surfaces and indoor environments are important sources of infectious spread within hospital and non-hospital facilities. Bacterial infections such as infections with Clostridioides (formerly Clostridium) difficile (C. difficile) and Staphylococcus aureus (S. aureus) and its antibiotic resistant strains continue to pose a significant risk to healthcare workers and patients. Additionally, the recent emergence of the coronavirus disease 2019 (COVID-19) pandemic, which is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), highlights the need for safe and effective methods to decontaminate surfaces to control infection spread in hospitals and the community. To address these critical needs, we tested a photocatalytic reactor decontamination method to disinfect contaminated surfaces in a hospital and a laboratory setting. By placing the reactor in a test hospital room, growth of S. aureus and C. difficile were significantly reduced compared with a control room. Additionally, using a model enveloped positive-sense single-stranded RNA virus, dengue virus type 2 (DENV2), we showed that the use of the photocatalytic reactor reduces viral infectivity. Collectively, the results demonstrate the potential utility of photocatalytic reactors in reducing the spread of highly contagious bacterial and viral infections through contaminated surfaces and environments.
Keywords: C. difficile; COVID-19; MRSA; RNA virus; Staphylococcus aureus; coronavirus; dengue virus; infection control; photocatalytic oxidation; photocatalytic reactor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
SARS-CoV-2 in environmental perspective: Occurrence, persistence, surveillance, inactivation and challenges.Chem Eng J. 2021 Feb 1;405:126893. doi: 10.1016/j.cej.2020.126893. Epub 2020 Sep 4. Chem Eng J. 2021. PMID: 32901196 Free PMC article.
-
The identification and epidemiology of meticillin-resistant Staphylococcus aureus and Clostridium difficile in patient rooms and the ward environment.BMC Infect Dis. 2013 Jul 24;13:342. doi: 10.1186/1471-2334-13-342. BMC Infect Dis. 2013. PMID: 23883171 Free PMC article.
-
Evaluation of an automated ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens in hospital rooms.BMC Infect Dis. 2010 Jul 8;10:197. doi: 10.1186/1471-2334-10-197. BMC Infect Dis. 2010. PMID: 20615229 Free PMC article.
-
Cleaning and disinfecting surfaces in hospitals and long-term care facilities for reducing hospital- and facility-acquired bacterial and viral infections: a systematic review.J Hosp Infect. 2022 Apr;122:9-26. doi: 10.1016/j.jhin.2021.12.017. Epub 2022 Jan 6. J Hosp Infect. 2022. PMID: 34998912 Review.
-
Clostridioides difficile infection (CDI) during the COVID-19 pandemic.Anaerobe. 2022 Apr;74:102518. doi: 10.1016/j.anaerobe.2022.102518. Epub 2022 Jan 19. Anaerobe. 2022. PMID: 35063599 Free PMC article. Review.
References
-
- Peyrony O., Marbeuf-Gueye C., Truong V., Giroud M., Rivière C., Khenissi K., Legay L., Simonetta M., Elezi A., Principe A., et al. Accuracy of Emergency Department Clinical Findings for Diagnosis of Coronavirus Disease 2019. Ann. Emerg. Med. 2020;76:405–412. doi: 10.1016/j.annemergmed.2020.05.022. - DOI - PMC - PubMed
-
- Magill S.S., Edwards J.R., Bamberg W., Beldavs Z.G., Dumyati G., Kainer M.A., Lynfield R., Maloney M., McAllister-Hollod L., Nadle J., et al. Multistate Point-Prevalence Survey of Health Care—Associated Infections. N. Engl. J. Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention The Direct Medical Costs of Healthcare-Associated Infections in U.S. Hospitals and the Benefits of Prevention. [(accessed on 25 October 2020)]; Available online: https://stacks.cdc.gov/view/cdc/11550.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

