Cell death pathways: intricate connections and disease implications
- PMID: 33439509
- PMCID: PMC7917554
- DOI: 10.15252/embj.2020106700
Cell death pathways: intricate connections and disease implications
Abstract
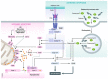
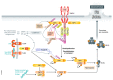
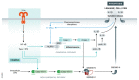
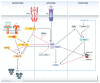
Various forms of cell death have been identified over the last decades with each relying on a different subset of proteins for the activation and execution of their respective pathway(s). In addition to the three best characterized pathways-apoptosis, necroptosis, and pyroptosis-other forms of regulated cell death including autophagy-dependent cell death (ADCD), mitochondrial permeability transition pore (MPTP)-mediated necrosis, parthanatos, NETosis and ferroptosis, and their relevance for organismal homeostasis are becoming better understood. Importantly, it is increasingly clear that none of these pathways operate alone. Instead, a more complex picture is emerging with many pathways sharing components and signaling principles. Finally, a number of cell death regulators are implicated in human diseases and represent attractive therapeutic targets. Therefore, better understanding of physiological and mechanistic aspects of cell death signaling should yield improved reagents for addressing unmet medical needs.
Keywords: RIPK1; apoptosis; caspase; necroptosis; pyroptosis.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease.Physiol Rev. 2019 Oct 1;99(4):1765-1817. doi: 10.1152/physrev.00022.2018. Physiol Rev. 2019. PMID: 31364924 Free PMC article. Review.
-
Beyond apoptosis: evidence of other regulated cell death pathways in the ovary throughout development and life.Hum Reprod Update. 2023 Jul 5;29(4):434-456. doi: 10.1093/humupd/dmad005. Hum Reprod Update. 2023. PMID: 36857094 Free PMC article. Review.
-
Regulated necrosis: the expanding network of non-apoptotic cell death pathways.Nat Rev Mol Cell Biol. 2014 Feb;15(2):135-47. doi: 10.1038/nrm3737. Nat Rev Mol Cell Biol. 2014. PMID: 24452471 Review.
-
Cell death mechanisms in eukaryotes.Cell Biol Toxicol. 2020 Apr;36(2):145-164. doi: 10.1007/s10565-019-09496-2. Epub 2019 Dec 9. Cell Biol Toxicol. 2020. PMID: 31820165 Review.
-
Pore formation in regulated cell death.EMBO J. 2020 Dec 1;39(23):e105753. doi: 10.15252/embj.2020105753. Epub 2020 Oct 30. EMBO J. 2020. PMID: 33124082 Free PMC article. Review.
Cited by
-
G protein coupled receptor in apoptosis and apoptotic cell clearance.FASEB Bioadv. 2024 Aug 6;6(9):289-297. doi: 10.1096/fba.2024-00067. eCollection 2024 Sep. FASEB Bioadv. 2024. PMID: 39399480 Free PMC article. Review.
-
Dissecting cell death pathways in fed-batch bioreactors.Biotechnol J. 2024 Jan;19(1):e2300257. doi: 10.1002/biot.202300257. Epub 2023 Dec 7. Biotechnol J. 2024. PMID: 38038229 Free PMC article.
-
New insights into regulatory cell death and acute pancreatitis.Heliyon. 2023 Jul 7;9(7):e18036. doi: 10.1016/j.heliyon.2023.e18036. eCollection 2023 Jul. Heliyon. 2023. PMID: 37519748 Free PMC article. Review.
-
Emerging roles of growth differentiation factor-15 in brain disorders (Review).Exp Ther Med. 2021 Nov;22(5):1270. doi: 10.3892/etm.2021.10705. Epub 2021 Sep 7. Exp Ther Med. 2021. PMID: 34594407 Free PMC article. Review.
-
Paraptosis-A Distinct Pathway to Cell Death.Int J Mol Sci. 2024 Oct 25;25(21):11478. doi: 10.3390/ijms252111478. Int J Mol Sci. 2024. PMID: 39519031 Free PMC article. Review.
References
-
- Bailey HH, Ripple G, Tutsch KD, Arzoomanian RZ, Alberti D, Feierabend C, Mahvi D, Schink J, Pomplun M, Mulcahy RT et al (1997) Phase I study of continuous‐infusion L‐S, R‐buthionine sulfoximine with intravenous melphalan. J Natl Cancer Inst 89: 1789–1796 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

