Placental growth factor promotes tumour desmoplasia and treatment resistance in intrahepatic cholangiocarcinoma
- PMID: 33431577
- PMCID: PMC8666816
- DOI: 10.1136/gutjnl-2020-322493
Placental growth factor promotes tumour desmoplasia and treatment resistance in intrahepatic cholangiocarcinoma
Abstract
Objective: Intrahepatic cholangiocarcinoma (ICC)-a rare liver malignancy with limited therapeutic options-is characterised by aggressive progression, desmoplasia and vascular abnormalities. The aim of this study was to determine the role of placental growth factor (PlGF) in ICC progression.
Design: We evaluated the expression of PlGF in specimens from ICC patients and assessed the therapeutic effect of genetic or pharmacologic inhibition of PlGF in orthotopically grafted ICC mouse models. We evaluated the impact of PlGF stimulation or blockade in ICC cells and cancer-associated fibroblasts (CAFs) using in vitro 3-D coculture systems.
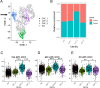
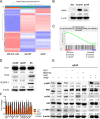
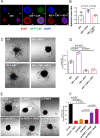
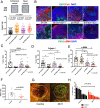
Results: PlGF levels were elevated in human ICC stromal cells and circulating blood plasma and were associated with disease progression. Single-cell RNA sequencing showed that the major impact of PlGF blockade in mice was enrichment of quiescent CAFs, characterised by high gene transcription levels related to the Akt pathway, glycolysis and hypoxia signalling. PlGF blockade suppressed Akt phosphorylation and myofibroblast activation in ICC-derived CAFs. PlGF blockade also reduced desmoplasia and tissue stiffness, which resulted in reopening of collapsed tumour vessels and improved blood perfusion, while reducing ICC cell invasion. Moreover, PlGF blockade enhanced the efficacy of standard chemotherapy in mice-bearing ICC. Conclusion PlGF blockade leads to a reduction in intratumorous hypoxia and metastatic dissemination, enhanced chemotherapy sensitivity and increased survival in mice-bearing aggressive ICC.
Keywords: cholangiocarcinoma; hepatic fibrosis.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: IC is an employee of STIMIT. TY has served in a consulting or advisory role for Bristol Myers Squibb. RKJ received honorarium from Amgen and consultant fees from Chugai, Ophthotech, Merck, SPARC, SynDevRx. RKJ owns equity in Accurius, Enlight, SPARC, and SynDevRx, and serves on the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund and Tekla World Healthcare Fund. AXZ is a consultant/advisory board member for Bayer. DGD received consultant fees from Bayer, Simcere, Surface Oncology and BMS and research grants from Bayer, Exelixis and BMS. No reagents or support from these companies was used for this study.
Figures


Similar articles
-
CAFs shape myeloid-derived suppressor cells to promote stemness of intrahepatic cholangiocarcinoma through 5-lipoxygenase.Hepatology. 2022 Jan;75(1):28-42. doi: 10.1002/hep.32099. Epub 2021 Dec 5. Hepatology. 2022. PMID: 34387870
-
Modulating PCGF4/BMI1 Stability Is an Efficient Metastasis-Regulatory Strategy Used by Distinct Subtypes of Cancer-Associated Fibroblasts in Intrahepatic Cholangiocarcinoma.Am J Pathol. 2024 Jul;194(7):1388-1404. doi: 10.1016/j.ajpath.2024.03.012. Epub 2024 Apr 24. Am J Pathol. 2024. PMID: 38670529
-
Fibroblastic FAP promotes intrahepatic cholangiocarcinoma growth via MDSCs recruitment.Neoplasia. 2019 Dec;21(12):1133-1142. doi: 10.1016/j.neo.2019.10.005. Epub 2019 Nov 20. Neoplasia. 2019. PMID: 31759251 Free PMC article.
-
The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma.Nat Rev Gastroenterol Hepatol. 2011 Nov 29;9(1):44-54. doi: 10.1038/nrgastro.2011.222. Nat Rev Gastroenterol Hepatol. 2011. PMID: 22143274 Review.
-
Periostin in intrahepatic cholangiocarcinoma: pathobiological insights and clinical implications.Exp Mol Pathol. 2014 Dec;97(3):515-24. doi: 10.1016/j.yexmp.2014.10.007. Epub 2014 Oct 28. Exp Mol Pathol. 2014. PMID: 25446840 Free PMC article. Review.
Cited by
-
PD-L1-directed PlGF/VEGF blockade synergizes with chemotherapy by targeting CD141+ cancer-associated fibroblasts in pancreatic cancer.Nat Commun. 2022 Oct 22;13(1):6292. doi: 10.1038/s41467-022-33991-6. Nat Commun. 2022. PMID: 36272973 Free PMC article.
-
Refining genetic and molecular classifications to facilitate breakthrough treatments in intrahepatic cholangiocarcinoma: are we there yet?Gut. 2023 Apr;72(4):608-610. doi: 10.1136/gutjnl-2022-327782. Epub 2022 Jun 30. Gut. 2023. PMID: 35772925 Free PMC article. No abstract available.
-
Placental growth factor mediates pathological uterine angiogenesis by activating the NFAT5-SGK1 signaling axis in the endometrium: implications for preeclampsia development.Biol Res. 2024 Aug 17;57(1):55. doi: 10.1186/s40659-024-00526-w. Biol Res. 2024. PMID: 39152497 Free PMC article.
-
Cancer-Associated Fibroblasts in Cholangiocarcinoma: Current Knowledge and Possible Implications for Therapy.J Clin Med. 2022 Nov 2;11(21):6498. doi: 10.3390/jcm11216498. J Clin Med. 2022. PMID: 36362726 Free PMC article. Review.
-
Comprehensive Analysis for Anti-Cancer Target-Indication Prioritization of Placental Growth Factor Inhibitor (PGF) by Use of Omics and Patient Survival Data.Biology (Basel). 2023 Jul 7;12(7):970. doi: 10.3390/biology12070970. Biology (Basel). 2023. PMID: 37508400 Free PMC article.
References
-
- Jusakul A, Cutcutache I, Yong CH, et al. . Whole-Genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov 2017;7:1116–35. 10.1158/2159-8290.CD-17-0368 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
