Antibody and Cell-Mediated Immune Responses Are Correlates of Protection against Influenza Infection in Vaccinated Older Adults
- PMID: 33430191
- PMCID: PMC7825602
- DOI: 10.3390/vaccines9010025
Antibody and Cell-Mediated Immune Responses Are Correlates of Protection against Influenza Infection in Vaccinated Older Adults
Abstract
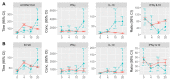
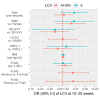
Despite efforts to design better vaccines for older adults, the risk for serious complications of influenza remains disproportionately high. Identifying correlates of vaccine effectiveness and understanding the heterogeneity of health outcomes in older adults are key to the vaccine development pipeline. We sought correlates of protection against laboratory-confirmed influenza illness (LCII) in a 4-year randomized trial of standard versus high-dose influenza vaccination of adults 65 years and older. To this end, we quantified serum hemagglutination-inhibition (HAI) titers and interferon-gamma (IFNγ) and interleukin-10 (IL-10) secretion by virus-challenged peripheral blood mononuclear cells. Of the 608 participants included, 26 developed either A/H3N2-(n = 17) or B-LCII (n = 9) at 10-20 weeks post-vaccination. Antibody titres for A/H3N2 at 4-weeks post-vaccination were significantly associated with protection against LCII, where every 1-standard deviation increase reduced the odds of A/H3N2-LCII by 53%. Although B-titres did not correlate with protection against B-LCII, the fold-increase in IFNγ:IL-10 ratios from pre- to 4-weeks post-vaccination was significantly associated with protection against B-LCII, where every 1-standard deviation increase reduced the odds by 71%. Our results suggest that both antibody and cell-mediated immune measures are valuable and potentially complementary correlates of protection against LCII in vaccinated older adults, although this may depend on the viral type causing infection.
Keywords: antibody; cell-mediated immunity; correlates of protection; influenza; older adults; vaccination.
Conflict of interest statement
J.E.M. reports payments to her institution for her participation in advisory, scientific, or data safety and monitoring boards from Sanofi, G.S.K., Pfizer, Merck, ResTORbio, and Medicago, and as a site lead clinical trials sponsored by VBI and Jansen, outside the submitted work. L.H. reports payments from Spring Discovery for her work in an advisory capacity. M.L. reports grant funding, in-kind supply of vaccines, or honoraria for participation in advisory boards, from Seqirus, Sanofi, Medicago, and Pfizer. M.K.A. reports payments from GSK (grant funding), Sanofi (grants, honoraria and consulting fees) and Pfizer (grants and honoraria), outside the submitted work. G.A.K. reports grants from the US National Institutes of Health and honoraria for participation in advisory boards from ResTORbio, Janssen and Spring Discovery. C.P.V. and G.P. report no conflicts of interest. The funders had no role in the design of the study, collection, analyses, or interpretation of data, the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Granzyme B: a double-edged sword in the response to influenza infection in vaccinated older adults.Front Aging. 2021;2:753767. doi: 10.3389/fragi.2021.753767. Epub 2021 Nov 11. Front Aging. 2021. PMID: 35441156 Free PMC article.
-
Key Determinants of Cell-Mediated Immune Responses: A Randomized Trial of High Dose Vs. Standard Dose Split-Virus Influenza Vaccine in Older Adults.Front Aging. 2021 May;2:649110. doi: 10.3389/fragi.2021.649110. Epub 2021 May 21. Front Aging. 2021. PMID: 35128529 Free PMC article.
-
Immunogenicity of AS03-adjuvanted and non-adjuvanted trivalent inactivated influenza vaccines in elderly adults: A Phase 3, randomized trial and post-hoc correlate of protection analysis.Hum Vaccin Immunother. 2016 Dec;12(12):3043-3055. doi: 10.1080/21645515.2016.1219809. Hum Vaccin Immunother. 2016. PMID: 27690762 Free PMC article. Clinical Trial.
-
T-Cell Immunity to Influenza in Older Adults: A Pathophysiological Framework for Development of More Effective Vaccines.Front Immunol. 2016 Feb 25;7:41. doi: 10.3389/fimmu.2016.00041. eCollection 2016. Front Immunol. 2016. PMID: 26941738 Free PMC article. Review.
-
Influenza vaccine responses in older adults.Ageing Res Rev. 2011 Jul;10(3):379-88. doi: 10.1016/j.arr.2010.10.008. Epub 2010 Nov 3. Ageing Res Rev. 2011. PMID: 21055484 Free PMC article. Review.
Cited by
-
Granzyme B: a double-edged sword in the response to influenza infection in vaccinated older adults.Front Aging. 2021;2:753767. doi: 10.3389/fragi.2021.753767. Epub 2021 Nov 11. Front Aging. 2021. PMID: 35441156 Free PMC article.
-
Immunosenescence and Altered Vaccine Efficiency in Older Subjects: A Myth Difficult to Change.Vaccines (Basel). 2022 Apr 13;10(4):607. doi: 10.3390/vaccines10040607. Vaccines (Basel). 2022. PMID: 35455356 Free PMC article. Review.
-
The effect of metformin on influenza vaccine responses in nondiabetic older adults: a pilot trial.Immun Ageing. 2023 May 2;20(1):18. doi: 10.1186/s12979-023-00343-x. Immun Ageing. 2023. PMID: 37131271 Free PMC article.
-
The role of cell-mediated immunity against influenza and its implications for vaccine evaluation.Front Immunol. 2022 Aug 16;13:959379. doi: 10.3389/fimmu.2022.959379. eCollection 2022. Front Immunol. 2022. PMID: 36052083 Free PMC article. Review.
-
Immunogenicity of adjuvanted versus high-dose inactivated influenza vaccines in older adults: a randomized clinical trial.Immun Ageing. 2023 Jul 1;20(1):30. doi: 10.1186/s12979-023-00355-7. Immun Ageing. 2023. PMID: 37393237 Free PMC article.
References
-
- Troeger C.E., Blacker B.F., Khalil I.A., Zimsen S.R.M., Albertson S.B., Abate D., Abdela J., Adhikari T.B., Aghayan S.A., Agrawal S., et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: An analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2019;7:69–89. doi: 10.1016/S2213-2600(18)30496-X. - DOI - PMC - PubMed
-
- CDC Weekly U.S. Influenza Surveillance Report (FluView) [(accessed on 5 May 2020)]; Available online: https://www.cdc.gov/flu/weekly/index.htm.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

