Combinatorial Drug Treatments Reveal Promising Anticytomegaloviral Profiles for Clinically Relevant Pharmaceutical Kinase Inhibitors (PKIs)
- PMID: 33430060
- PMCID: PMC7826512
- DOI: 10.3390/ijms22020575
Combinatorial Drug Treatments Reveal Promising Anticytomegaloviral Profiles for Clinically Relevant Pharmaceutical Kinase Inhibitors (PKIs)
Abstract
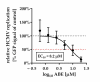
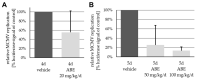
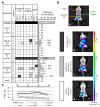
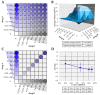
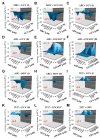
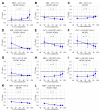
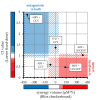
Human cytomegalovirus (HCMV) is a human pathogenic herpesvirus associated with a variety of clinical symptoms. Current antiviral therapy is not always effective, so that improved drug classes and drug-targeting strategies are needed. Particularly host-directed antivirals, including pharmaceutical kinase inhibitors (PKIs), may help to overcome problems of drug resistance. Here, we focused on utilizing a selection of clinically relevant PKIs and determined their anticytomegaloviral efficacies. Particularly, PKIs directed to host or viral cyclin-dependent kinases, i.e., abemaciclib, LDC4297 and maribavir, exerted promising profiles against human and murine cytomegaloviruses. The anti-HCMV in vitro activity of the approved anti-cancer drug abemaciclib was confirmed in vivo using our luciferase-based murine cytomegalovirus (MCMV) animal model in immunocompetent mice. To assess drug combinations, we applied the Bliss independence checkerboard and Loewe additivity fixed-dose assays in parallel. Results revealed that (i) both affirmative approaches provided valuable information on anti-CMV drug efficacies and interactions, (ii) the analyzed combinations comprised additive, synergistic or antagonistic drug interactions consistent with the drugs' antiviral mode-of-action, (iii) the selected PKIs, especially LDC4297, showed promising inhibitory profiles, not only against HCMV but also other α-, β- and γ-herpesviruses, and specifically, (iv) the combination treatment with LDC4297 and maribavir revealed a strong synergism against HCMV, which might open doors towards novel clinical options in the near future. Taken together, this study highlights the potential of therapeutic drug combinations of current developmental/preclinical PKIs.
Keywords: activity in vitro and in vivo; antiviral drugs; combinatorial drug analyses; human cytomegalovirus; new synergistic combinations; pharmaceutical kinase inhibitors (PKIs).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
In vitro evaluation of current and novel antivirals in combination against human cytomegalovirus.Antiviral Res. 2018 Oct;158:255-263. doi: 10.1016/j.antiviral.2018.08.015. Epub 2018 Aug 25. Antiviral Res. 2018. PMID: 30153445
-
Cyclin-Dependent Kinases (CDKs) and the Human Cytomegalovirus-Encoded CDK Ortholog pUL97 Represent Highly Attractive Targets for Synergistic Drug Combinations.Int J Mol Sci. 2022 Feb 24;23(5):2493. doi: 10.3390/ijms23052493. Int J Mol Sci. 2022. PMID: 35269635 Free PMC article.
-
In vivo proof-of-concept for two experimental antiviral drugs, both directed to cellular targets, using a murine cytomegalovirus model.Antiviral Res. 2019 Jan;161:63-69. doi: 10.1016/j.antiviral.2018.11.008. Epub 2018 Nov 17. Antiviral Res. 2019. PMID: 30452929
-
Clinical development of letermovir and maribavir: Overview of human cytomegalovirus drug resistance.Antiviral Res. 2019 Mar;163:91-105. doi: 10.1016/j.antiviral.2019.01.011. Epub 2019 Jan 25. Antiviral Res. 2019. PMID: 30690043 Review.
-
[Modern ethiotropic chemotherapy of human cytomegalovirus infection: clinical effectiveness, molecular mechanism of action, drug resistance, new trends and prospects. Part 2.].Vopr Virusol. 2018;63(6):250-260. doi: 10.18821/0507-4088-2018-63-6-250-260. Vopr Virusol. 2018. PMID: 30641020 Review. Russian.
Cited by
-
The Oligomeric Assemblies of Cytomegalovirus Core Nuclear Egress Proteins Are Associated with Host Kinases and Show Sensitivity to Antiviral Kinase Inhibitors.Viruses. 2022 May 11;14(5):1021. doi: 10.3390/v14051021. Viruses. 2022. PMID: 35632762 Free PMC article.
-
Development of a PROTAC-Based Targeting Strategy Provides a Mechanistically Unique Mode of Anti-Cytomegalovirus Activity.Int J Mol Sci. 2021 Nov 27;22(23):12858. doi: 10.3390/ijms222312858. Int J Mol Sci. 2021. PMID: 34884662 Free PMC article.
-
'Getting Better'-Is It a Feasible Strategy of Broad Pan-Antiherpesviral Drug Targeting by Using the Nuclear Egress-Directed Mechanism?Int J Mol Sci. 2024 Feb 29;25(5):2823. doi: 10.3390/ijms25052823. Int J Mol Sci. 2024. PMID: 38474070 Free PMC article. Review.
-
Understanding the Cytomegalovirus Cyclin-Dependent Kinase Ortholog pUL97 as a Multifaceted Regulator and an Antiviral Drug Target.Cells. 2024 Aug 13;13(16):1338. doi: 10.3390/cells13161338. Cells. 2024. PMID: 39195228 Free PMC article. Review.
-
Interactions of plumbagin with five common antibiotics against Staphylococcus aureus in vitro.PLoS One. 2024 Jan 26;19(1):e0297493. doi: 10.1371/journal.pone.0297493. eCollection 2024. PLoS One. 2024. PMID: 38277418 Free PMC article.
References
-
- Mocarski E.S., Shenk T., Griffiths P.D., Pass R.F. Fields Virology. 6th ed. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2013. Cytomegaloviruses; pp. 1960–2014.
-
- Sever J.L., Rakusan T.A., Ellaurie M., Frenkel N., Wyatt L.S., Campos J.M., O’Donnell R.M., Price M.V. Coinfection with herpesviruses in young children of HIV-infected women. Pediatr. AIDS HIV Infect. 1995;6:75–82. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

