Anti-IL6 treatment of serious COVID-19 disease: A monocentric retrospective experience
- PMID: 33429732
- PMCID: PMC7793456
- DOI: 10.1097/MD.0000000000023582
Anti-IL6 treatment of serious COVID-19 disease: A monocentric retrospective experience
Abstract
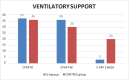
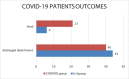
COVID-19 is causing a high influx of patients suffering from serious respiratory complications leading the necessity to find effective therapies. These patients seem to present with cytokine perturbation and high levels of IL6. Tocilizumab and sarilumab could be effective in this condition.We retrospectively collected data about 112 consecutive hospitalized in a single center.Fifty (IL6 group) treated with tocilizumab (8 mg/kg intravenously [IV], 2 infusions 12 hours apart) or sarilumab 400 mg IV once and 62 treated with the standard of care but not anti-cytokine drugs (CONTROL group).To determine whether anti-IL6 drugs are effective in improving prognosis and reducing hospitalization times and mortality in COVID-19 pneumonia.To date 84% (42/50) of IL6 group patients have already been discharged and only 2/50 are still recovered and intubated in intensive care. Six/fifty patients (12%) died: 5/6 due to severe respiratory failure within a framework of severe acute respiratory distress syndrome (ARDS), 1 suffered an acute myocardial infarction, and 1 died of massive pulmonary thromboembolism. There were no adverse treatment events or infectious complications. Compared to the CONTROL group they showed a lower mortality rate (12% versus 43%), for the same number of complications and days of hospitalization.Anti-IL6 drugs seem to be effective in the treatment of medium to severe forms of COVID-19 pneumonia reducing the risk of mortality due to multi-organ failure, acting at the systemic level and reducing inflammation levels and therefore microvascular complications. However, it is essential to identify the best time for treatment, which, if delayed, is rendered useless as well as counterproductive. Further studies and ongoing clinical trials will help us to better define patients eligible as candidates for more aggressive intervention.
Copyright © 2021 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Sarilumab versus standard of care for the early treatment of COVID-19 pneumonia in hospitalized patients: SARTRE: a structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Sep 16;21(1):794. doi: 10.1186/s13063-020-04633-3. Trials. 2020. PMID: 32938496 Free PMC article.
-
A Phase 3 Open-label, Randomized, Controlled Study to Evaluate the Efficacy and Safety of Intravenously Administered Ravulizumab Compared with Best Supportive Care in Patients with COVID-19 Severe Pneumonia, Acute Lung Injury, or Acute Respiratory Distress Syndrome: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Jul 13;21(1):639. doi: 10.1186/s13063-020-04548-z. Trials. 2020. PMID: 32660611 Free PMC article.
-
Subcutaneous Sarilumab in hospitalised patients with moderate-severe COVID-19 infection compared to the standard of care (SARCOVID): a structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Sep 9;21(1):772. doi: 10.1186/s13063-020-04588-5. Trials. 2020. PMID: 32907638 Free PMC article.
-
Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy.Autoimmun Rev. 2020 Jul;19(7):102568. doi: 10.1016/j.autrev.2020.102568. Epub 2020 May 3. Autoimmun Rev. 2020. PMID: 32376398 Free PMC article. Review.
-
Rational Use of Tocilizumab in the Treatment of Novel Coronavirus Pneumonia.Clin Drug Investig. 2020 Jun;40(6):511-518. doi: 10.1007/s40261-020-00917-3. Clin Drug Investig. 2020. PMID: 32337664 Free PMC article. Review.
Cited by
-
COVID-19 and Rheumatoid Arthritis Crosstalk: Emerging Association, Therapeutic Options and Challenges.Cells. 2021 Nov 24;10(12):3291. doi: 10.3390/cells10123291. Cells. 2021. PMID: 34943795 Free PMC article. Review.
-
Modulation of Covid-19 cytokine storm by tocilizumab.J Med Virol. 2022 Mar;94(3):823-828. doi: 10.1002/jmv.27380. Epub 2021 Oct 23. J Med Virol. 2022. PMID: 34617604 Free PMC article.
-
Difficulty in management of acute invasive fungal rhinosinusitis in Indonesia during the COVID-19 pandemic: A case report.Respir Med Case Rep. 2023 Sep 16;46:101916. doi: 10.1016/j.rmcr.2023.101916. eCollection 2023. Respir Med Case Rep. 2023. PMID: 38046461 Free PMC article.
-
Animal models for SARS-CoV-2 and SARS-CoV-1 pathogenesis, transmission and therapeutic evaluation.World J Virol. 2022 Jan 25;11(1):40-56. doi: 10.5501/wjv.v11.i1.40. World J Virol. 2022. PMID: 35117970 Free PMC article. Review.
-
cGAS deficiency enhances inflammasome activation in macrophages and inflammatory pathology in pristane-induced lupus.Front Immunol. 2022 Dec 16;13:1010764. doi: 10.3389/fimmu.2022.1010764. eCollection 2022. Front Immunol. 2022. PMID: 36591278 Free PMC article.
References
-
- Cai X. An Insight of Comparison Between COVID-19 (2019-nCoVdisease) and SARS in Pathology and Pathogenesis. Shanghai, PR China: Hanbio Research Center, Hanbio Tech Co. Ltd.; 2020.
-
- Chen C, Zhang XR, Ju ZY, et al. Advances in the research of mechanism and related immunotherapy on the cytokine storm induced by coronavirus disease 2019. Zhonghua Shao Shang Za Zhi 2020;36:471–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

