Why Inclusion Matters for Alzheimer's Disease Biomarker Discovery in Plasma
- PMID: 33427747
- PMCID: PMC9126484
- DOI: 10.3233/JAD-201318
Why Inclusion Matters for Alzheimer's Disease Biomarker Discovery in Plasma
Erratum in
-
Erratum Why Inclusion Matters for Alzheimer's Disease Biomarker Discovery in Plasma.J Alzheimers Dis. 2021;80(4):1739. doi: 10.3233/JAD-219318. J Alzheimers Dis. 2021. PMID: 33843689 No abstract available.
Abstract
Background: African American/Black adults have a disproportionate incidence of Alzheimer's disease (AD) and are underrepresented in biomarker discovery efforts.
Objective: This study aimed to identify potential diagnostic biomarkers for AD using a combination of proteomics and machine learning approaches in a cohort that included African American/Black adults.
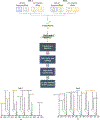
Methods: We conducted a discovery-based plasma proteomics study on plasma samples (N = 113) obtained from clinically diagnosed AD and cognitively normal adults that were self-reported African American/Black or non-Hispanic White. Sets of differentially-expressed proteins were then classified using a support vector machine (SVM) to identify biomarker candidates.
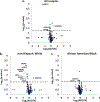
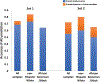
Results: In total, 740 proteins were identified of which, 25 differentially-expressed proteins in AD came from comparisons within a single racial and ethnic background group. Six proteins were differentially-expressed in AD regardless of racial and ethnic background. Supervised classification by SVM yielded an area under the curve (AUC) of 0.91 and accuracy of 86%for differentiating AD in samples from non-Hispanic White adults when trained with differentially-expressed proteins unique to that group. However, the same model yielded an AUC of 0.49 and accuracy of 47%for differentiating AD in samples from African American/Black adults. Other covariates such as age, APOE4 status, sex, and years of education were found to improve the model mostly in the samples from non-Hispanic White adults for classifying AD.
Conclusion: These results demonstrate the importance of study designs in AD biomarker discovery, which must include diverse racial and ethnic groups such as African American/Black adults to develop effective biomarkers.
Keywords: African American; Alzheimer’s disease; Black; biomarker; discovery; disparities; machine learning; plasma; proteomics; race.
Figures

Similar articles
-
Exposing the Brain Proteomic Signatures of Alzheimer's Disease in Diverse Racial Groups: Leveraging Multiple Data Sets and Machine Learning.J Proteome Res. 2022 Apr 1;21(4):1095-1104. doi: 10.1021/acs.jproteome.1c00966. Epub 2022 Mar 11. J Proteome Res. 2022. PMID: 35276041 Free PMC article.
-
Inclusion of African American/Black adults in a pilot brain proteomics study of Alzheimer's disease.Neurobiol Dis. 2020 Dec;146:105129. doi: 10.1016/j.nbd.2020.105129. Epub 2020 Oct 10. Neurobiol Dis. 2020. PMID: 33049317 Free PMC article.
-
Dataset of why inclusion matters for Alzheimer's disease biomarker discovery in plasma.Data Brief. 2021 Mar 1;35:106923. doi: 10.1016/j.dib.2021.106923. eCollection 2021 Apr. Data Brief. 2021. PMID: 33786345 Free PMC article.
-
Quantification of race/ethnicity representation in Alzheimer's disease neuroimaging research in the USA: a systematic review.Commun Med (Lond). 2023 Jul 25;3(1):101. doi: 10.1038/s43856-023-00333-6. Commun Med (Lond). 2023. PMID: 37491471 Free PMC article. Review.
-
Assessment of the Inclusion of Racial/Ethnic Minority, Female, and Older Individuals in Vaccine Clinical Trials.JAMA Netw Open. 2021 Feb 1;4(2):e2037640. doi: 10.1001/jamanetworkopen.2020.37640. JAMA Netw Open. 2021. PMID: 33606033 Free PMC article. Review.
Cited by
-
Advances, obstacles, and opportunities for machine learning in proteomics.Cell Rep Phys Sci. 2022 Oct 19;3(10):101069. doi: 10.1016/j.xcrp.2022.101069. Epub 2022 Sep 22. Cell Rep Phys Sci. 2022. PMID: 36381226 Free PMC article.
-
Alzheimer's disease CSF biomarkers correlate with early pathology and alterations in neuronal and glial gene expression.medRxiv [Preprint]. 2024 Jun 13:2024.06.11.24308706. doi: 10.1101/2024.06.11.24308706. medRxiv. 2024. Update in: Alzheimers Dement. 2024 Oct;20(10):7090-7103. doi: 10.1002/alz.14194 PMID: 38947015 Free PMC article. Updated. Preprint.
-
Exposing the Brain Proteomic Signatures of Alzheimer's Disease in Diverse Racial Groups: Leveraging Multiple Data Sets and Machine Learning.J Proteome Res. 2022 Apr 1;21(4):1095-1104. doi: 10.1021/acs.jproteome.1c00966. Epub 2022 Mar 11. J Proteome Res. 2022. PMID: 35276041 Free PMC article.
-
Prognosis of Alzheimer's Disease Using Quantitative Mass Spectrometry of Human Blood Plasma Proteins and Machine Learning.Int J Mol Sci. 2022 Jul 18;23(14):7907. doi: 10.3390/ijms23147907. Int J Mol Sci. 2022. PMID: 35887259 Free PMC article.
-
Demographic and Clinical Characteristics Associated With Serum GFAP Levels in an Ethnically Diverse Cohort.Neurology. 2023 Oct 10;101(15):e1531-e1541. doi: 10.1212/WNL.0000000000207706. Neurology. 2023. PMID: 37813589 Free PMC article.
References
-
- Lines L, Sherif N, Wiener J (2014) Racial and ethnic disparities among individuals with Alzheimer’s disease in the United States: A literature review. RTI Press, RTI Press Publication No. RR-0024–1412.
-
- (2020) 2020. Alzheimer’s disease facts and figures. Alzheimers Dement 16, 391–460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

