The application of bone marrow mesenchymal stem cells and biomaterials in skeletal muscle regeneration
- PMID: 33426231
- PMCID: PMC7770413
- DOI: 10.1016/j.reth.2020.11.002
The application of bone marrow mesenchymal stem cells and biomaterials in skeletal muscle regeneration
Abstract
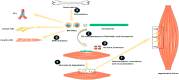
Skeletal muscle injuries have bothered doctors and caused great burdens to the public medical insurance system for a long time. Once injured, skeletal muscles usually go through the processes of inflammation, repairing and remodeling. If repairing and remodeling stages are out of balance, scars will be formed to replace injured skeletal muscles. At present, clinicians usually use conventional methods to restore the injured skeletal muscles, such as flap transplantation. However, flap transplantation sometimes needs to sacrifice healthy autologous tissues and will bring extra harm to patients. In recent years, stem cells-based tissue engineering provides us new treatment ideas for skeletal muscle injuries. Stem cells are cells with multiple differentiation potential and have ability to differentiate into adult cells under special condition. Skeletal muscle tissues also have stem cells, called satellite cells, but they are in small amount and new muscle fibers that derived from them may not be enough to replace injured fibers. Bone marrow mesenchymal stem cells (BM-MSCs) could promote musculoskeletal tissue regeneration and activate the myogenic differentiation of satellite cells. Biomaterial is another important factor to promote tissue regeneration and greatly enhance physiological activities of stem cells in vivo. The combined use of stem cells and biomaterials will gradually become a mainstream to restore injured skeletal muscles in the future. This review article mainly focuses on the review of research about the application of BM-MSCs and several major biomaterials in skeletal muscle regeneration over the past decades.
Keywords: 3D-ECM, three dimensional extracellular matrix; ASCs, adipose stem cells; BDNF, brain derived neurotrophic factor; BM-MSCs; BM-MSCs, bone marrow mesenchymal stem cells; Biomaterial; CREB, cAMP- response element binding protein; DPSCs, dental pulp stem cells; Differentiation; ECM, extracellular matrix; ECs, endothelial cells; EGF, epidermal growth factor; FGF, fibroblast growth factor; FGF-2, fibroblast growth factor-2; GCSF, granulocyte colony-stimulating factor; GDNF, glial derived neurotrophic factor; GPT, gelatin-poly(ethylene glycol)- tyramine; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor-1; IL, interleukin; LIF, leukemia inhibitory factor; MRF, myogenic muscle factor; NSAIDs, non-steroidal drugs; PDGF-BB, platelet derived growth factor-BB; PGE2, prostaglandin E2; PRP, platelet rich plasma; S1P, sphingosine 1-phosphate; SDF-1, stromal cell derived factor-1; Skeletal muscle injury; TGF-β, transforming growth factor-β; Tissue regeneration; TrkB, tyrosine kinaseB; VEGF, vascular endothelial growth factor; VML, volumetric muscle loss.
© 2020 The Japanese Society for Regenerative Medicine. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
Similar articles
-
Mesenchymal stromal cells for bone sarcoma treatment: Roadmap to clinical practice.J Bone Oncol. 2019 Mar 19;16:100231. doi: 10.1016/j.jbo.2019.100231. eCollection 2019 Jun. J Bone Oncol. 2019. PMID: 30956944 Free PMC article. Review.
-
Regenerative medicine strategies for hair growth and regeneration: A narrative review of literature.Regen Ther. 2022 Oct 31;21:527-539. doi: 10.1016/j.reth.2022.10.005. eCollection 2022 Dec. Regen Ther. 2022. PMID: 36382136 Free PMC article. Review.
-
Combined use of bone marrow-derived mesenchymal stromal cells (BM-MSCs) and platelet rich plasma (PRP) stimulates proliferation and differentiation of myoblasts in vitro: new therapeutic perspectives for skeletal muscle repair/regeneration.Cell Tissue Res. 2018 Jun;372(3):549-570. doi: 10.1007/s00441-018-2792-3. Epub 2018 Feb 5. Cell Tissue Res. 2018. PMID: 29404727
-
The factors present in regenerating muscles impact bone marrow-derived mesenchymal stromal/stem cell fusion with myoblasts.Stem Cell Res Ther. 2019 Nov 21;10(1):343. doi: 10.1186/s13287-019-1444-1. Stem Cell Res Ther. 2019. PMID: 31753006 Free PMC article.
-
Periosteum and development of the tissue-engineered periosteum for guided bone regeneration.J Orthop Translat. 2022 Feb 16;33:41-54. doi: 10.1016/j.jot.2022.01.002. eCollection 2022 Mar. J Orthop Translat. 2022. PMID: 35228996 Free PMC article. Review.
Cited by
-
[A dual-crosslinked injectable hydrogel derived from muscular decellularized matrix promoting myoblasts proliferation and myogenic differentiation].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2023 Dec 15;37(12):1514-1522. doi: 10.7507/1002-1892.202306054. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2023. PMID: 38130196 Free PMC article. Chinese.
-
Differential responses to aging amongst the transcriptome and proteome of mesenchymal progenitor populations.Res Sq [Preprint]. 2023 Dec 15:rs.3.rs-3755129. doi: 10.21203/rs.3.rs-3755129/v1. Res Sq. 2023. Update in: J Gerontol A Biol Sci Med Sci. 2024 Sep 1;79(9):glae147. doi: 10.1093/gerona/glae147 PMID: 38168272 Free PMC article. Updated. Preprint.
-
Mesenchymal Stem Cells in Soft Tissue Regenerative Medicine: A Comprehensive Review.Medicina (Kaunas). 2023 Aug 10;59(8):1449. doi: 10.3390/medicina59081449. Medicina (Kaunas). 2023. PMID: 37629738 Free PMC article. Review.
-
Comparison of CD146 +/- mesenchymal stem cells in improving premature ovarian failure.Stem Cell Res Ther. 2022 Jun 21;13(1):267. doi: 10.1186/s13287-022-02916-x. Stem Cell Res Ther. 2022. PMID: 35729643 Free PMC article.
-
Therapeutic Mesenchymal Stem/Stromal Cells: Value, Challenges and Optimization.Front Cell Dev Biol. 2022 Jan 14;9:716853. doi: 10.3389/fcell.2021.716853. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35096805 Free PMC article. Review.
References
-
- Sicari B.M., Dearth C.L., Badylak S.F. Tissue engineering and regenerative medicine approaches to enhance the functional response to skeletal muscle injury. Anat Rec (Hoboken) 2014;297(1):51–64. - PubMed
-
- Caseiro A.R., Pereira T., Bártolo P.J., Santos J.D., Luís A.L., Mauricio A. Mesenchymal stem cells and biomaterials systems – perspectives for skeletal muscle tissue repair and regeneration. Procedia Eng. 2015;110:90–97.
-
- Wang Y.X., Rudnicki M.A. Satellite cells, the engines of muscle repair. Nat Rev Mol Cell Biol. 2011;13(2):127–133. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

