Short-term overnutrition induces white adipose tissue insulin resistance through sn-1,2-diacylglycerol/PKCε/insulin receptor Thr1160 phosphorylation
- PMID: 33411692
- PMCID: PMC7934919
- DOI: 10.1172/jci.insight.139946
Short-term overnutrition induces white adipose tissue insulin resistance through sn-1,2-diacylglycerol/PKCε/insulin receptor Thr1160 phosphorylation
Abstract
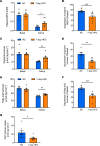
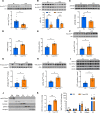
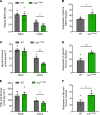
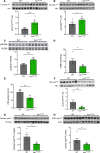
White adipose tissue (WAT) insulin action has critical anabolic function and is dysregulated in overnutrition. However, the mechanism of short-term high-fat diet-induced (HFD-induced) WAT insulin resistance (IR) is poorly understood. Based on recent evidences, we hypothesize that a short-term HFD causes WAT IR through plasma membrane (PM) sn-1,2-diacylglycerol (sn-1,2-DAG) accumulation, which promotes protein kinase C-ε (PKCε) activation to impair insulin signaling by phosphorylating insulin receptor (Insr) Thr1160. To test this hypothesis, we assessed WAT insulin action in 7-day HFD-fed versus regular chow diet-fed rats during a hyperinsulinemic-euglycemic clamp. HFD feeding caused WAT IR, reflected by impaired insulin-mediated WAT glucose uptake and lipolysis suppression. These changes were specifically associated with PM sn-1,2-DAG accumulation, higher PKCε activation, and impaired insulin-stimulated Insr Tyr1162 phosphorylation. In order to examine the role of Insr Thr1160 phosphorylation in mediating lipid-induced WAT IR, we examined these same parameters in InsrT1150A mice (mouse homolog for human Thr1160) and found that HFD feeding induced WAT IR in WT control mice but not in InsrT1150A mice. Taken together, these data demonstrate the importance of the PM sn-1,2-DAG/PKCε/Insr Thr1160 phosphorylation pathway in mediating lipid-induced WAT IR and represent a potential therapeutic target to improve WAT insulin sensitivity.
Keywords: Adipose tissue; Endocrinology; Glucose metabolism; Insulin signaling; Metabolism.
Conflict of interest statement
Figures
Similar articles
-
Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance.J Clin Invest. 2016 Nov 1;126(11):4361-4371. doi: 10.1172/JCI86013. Epub 2016 Oct 17. J Clin Invest. 2016. PMID: 27760050 Free PMC article.
-
Ceramide synthesis inhibitors prevent lipid-induced insulin resistance through the DAG-PKCε-insulin receptorT1150 phosphorylation pathway.Cell Rep. 2024 Oct 22;43(10):114746. doi: 10.1016/j.celrep.2024.114746. Epub 2024 Sep 19. Cell Rep. 2024. PMID: 39302831
-
Unsuppressed lipolysis in adipocytes is linked with enhanced gluconeogenesis and altered bile acid physiology in Insr(P1195L/+) mice fed high-fat-diet.Sci Rep. 2015 Nov 30;5:17565. doi: 10.1038/srep17565. Sci Rep. 2015. PMID: 26615883 Free PMC article.
-
Neural innervation of white adipose tissue and the control of lipolysis.Front Neuroendocrinol. 2014 Oct;35(4):473-93. doi: 10.1016/j.yfrne.2014.04.001. Epub 2014 Apr 13. Front Neuroendocrinol. 2014. PMID: 24736043 Free PMC article. Review.
-
Diacylglycerol activation of protein kinase Cε and hepatic insulin resistance.Cell Metab. 2012 May 2;15(5):574-84. doi: 10.1016/j.cmet.2012.03.005. Cell Metab. 2012. PMID: 22560210 Free PMC article. Review.
Cited by
-
Pathophysiological Mechanisms in Non-Alcoholic Fatty Liver Disease: From Drivers to Targets.Biomedicines. 2021 Dec 26;10(1):46. doi: 10.3390/biomedicines10010046. Biomedicines. 2021. PMID: 35052726 Free PMC article. Review.
-
High-fat-diet-induced hepatic insulin resistance per se attenuates murine de novo lipogenesis.iScience. 2024 Oct 15;27(11):111175. doi: 10.1016/j.isci.2024.111175. eCollection 2024 Nov 15. iScience. 2024. PMID: 39524330 Free PMC article.
-
Addressing Post-Acute COVID-19 Syndrome in Cancer Patients, from Visceral Obesity and Myosteatosis to Systemic Inflammation: Implications in Cardio-Onco-Metabolism.Biomedicines. 2024 Jul 24;12(8):1650. doi: 10.3390/biomedicines12081650. Biomedicines. 2024. PMID: 39200115 Free PMC article. Review.
-
Impact of Lipids on Insulin Resistance: Insights from Human and Animal Studies.Drug Des Devel Ther. 2024 Jul 31;18:3337-3360. doi: 10.2147/DDDT.S468147. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39100221 Free PMC article. Review.
-
Research Progress on the Mechanism Between Polycystic Ovary Syndrome and Abnormal Endometrium.Front Physiol. 2021 Dec 17;12:788772. doi: 10.3389/fphys.2021.788772. eCollection 2021. Front Physiol. 2021. PMID: 34975540 Free PMC article. Review.
References
-
- Bodis K, Roden M. Energy metabolism of white adipose tissue and insulin resistance in humans. Eur J Clin Invest. 2018;48(11):13017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

