Blocking the IL-1 receptor reduces cardiac transplant ischemia and reperfusion injury and mitigates CMV-accelerated chronic rejection
- PMID: 33405337
- PMCID: PMC11330275
- DOI: 10.1111/ajt.16149
Blocking the IL-1 receptor reduces cardiac transplant ischemia and reperfusion injury and mitigates CMV-accelerated chronic rejection
Abstract
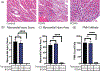
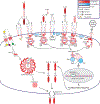
Ischemia-reperfusion injury (IRI) is an important risk factor for accelerated cardiac allograft rejection and graft dysfunction . Utilizing a rat heart isogeneic transplant model, we identified inflammatory pathways involved in IRI in order to identify therapeutic targets involved in disease. Pathway analyses identified several relevant targets, including cytokine signaling by the IL-1 receptor (IL-1R) pathway and inflammasome activation. To investigate the role of IL-1R signaling pathways during IRI, we treated syngeneic cardiac transplant recipients at 1-hour posttransplant with Anakinra, a US Food and Drug Administration (FDA)-approved IL-1R antagonist; or parthenolide, a caspase-1 and nuclear factor kappa-light-chain-enhancer of activated B cells inhibitor that blocks IL-1β maturation. Both Anakinra and parthenolide significantly reduced graft inflammation and cellular recruitment in the treated recipients relative to nontreated controls. Anakinra treatment administered at 1-hour posttransplant to recipients of cardiac allografts from CMV-infected donors significantly increased the time to rejection and reduced viral loads at rejection. Our results indicate that reducing IRI by blocking IL-1Rsignaling pathways with Anakinra or inflammasome activity with parthenolide provides a promising approach for extending survival of cardiac allografts from CMV-infected donors.
Keywords: animal models; basic (laboratory) research/science; complication: infectious; heart (allograft) function/dysfunction; heart transplantation/cardiology; immune regulation; immunobiology; infection and infectious agents – viral: cytomegalovirus (CMV); infectious disease.
© 2020 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the
Figures
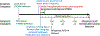


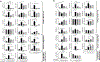
Similar articles
-
Rat Cytomegalovirus Vaccine Prevents Accelerated Chronic Rejection in CMV-Naïve Recipients of Infected Donor Allograft Hearts.Am J Transplant. 2015 Jul;15(7):1805-16. doi: 10.1111/ajt.13188. Epub 2015 Mar 12. Am J Transplant. 2015. PMID: 25766876 Free PMC article.
-
Angiopoietin-2 inhibition prevents transplant ischemia-reperfusion injury and chronic rejection in rat cardiac allografts.Am J Transplant. 2014 May;14(5):1096-108. doi: 10.1111/ajt.12672. Epub 2014 Apr 7. Am J Transplant. 2014. PMID: 24708486
-
Novel Application of Localized Nanodelivery of Anti-Interleukin-6 Protects Organ Transplant From Ischemia-Reperfusion Injuries.Am J Transplant. 2017 Sep;17(9):2326-2337. doi: 10.1111/ajt.14266. Epub 2017 Apr 18. Am J Transplant. 2017. PMID: 28296000 Free PMC article.
-
Cytomegalovirus infection and cardiac allograft vasculopathy.Transpl Infect Dis. 1999 Jun;1(2):115-26. doi: 10.1034/j.1399-3062.1999.010205.x. Transpl Infect Dis. 1999. PMID: 11428979 Review.
-
Interleukin-6: An Important Mediator of Allograft Injury.Transplantation. 2020 Dec;104(12):2497-2506. doi: 10.1097/TP.0000000000003249. Transplantation. 2020. PMID: 32235253 Review.
Cited by
-
Downregulation of the enhancer of zeste homolog 1 transcriptional factor predicts poor prognosis of triple-negative breast cancer patients.PeerJ. 2022 Jul 12;10:e13708. doi: 10.7717/peerj.13708. eCollection 2022. PeerJ. 2022. PMID: 35846880 Free PMC article.
-
Targeting IL-6 to prevent cardiac allograft rejection.Am J Transplant. 2022 Dec;22 Suppl 4(Suppl 4):12-17. doi: 10.1111/ajt.17206. Am J Transplant. 2022. PMID: 36453706 Free PMC article. Review.
-
Transmission of NLRP3-IL-1β Signals in Cerebral Ischemia and Reperfusion Injury: from Microglia to Adjacent Neuron and Endothelial Cells via IL-1β/IL-1R1/TRAF6.Mol Neurobiol. 2023 May;60(5):2749-2766. doi: 10.1007/s12035-023-03232-y. Epub 2023 Jan 30. Mol Neurobiol. 2023. PMID: 36717480
-
The Role of NLRP3 Inflammasome in Diabetic Cardiomyopathy and Its Therapeutic Implications.Oxid Med Cell Longev. 2022 Sep 6;2022:3790721. doi: 10.1155/2022/3790721. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36111168 Free PMC article. Review.
References
-
- Costello JP, Mohanakumar T, Nath DS. Mechanisms of chronic cardiac allograft rejection. Texas Hear Inst J. 2013;40(4):395–399. https://pubmed.ncbi.nlm.nih.gov/24082367. - PMC - PubMed

