Synthesis, Tumor Specificity, and Photosensitizing Efficacy of Erlotinib-Conjugated Chlorins and Bacteriochlorins: Identification of a Highly Effective Candidate for Photodynamic Therapy of Cancer
- PMID: 33400524
- PMCID: PMC9125565
- DOI: 10.1021/acs.jmedchem.0c01735
Synthesis, Tumor Specificity, and Photosensitizing Efficacy of Erlotinib-Conjugated Chlorins and Bacteriochlorins: Identification of a Highly Effective Candidate for Photodynamic Therapy of Cancer
Abstract
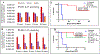
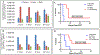
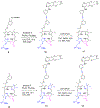
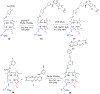
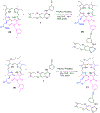
Erlotinib was covalently linked to 3-(1'-hexyloxy)ethyl-3-devinylpyropheophorbide-a (HPPH) and structurally related chlorins and bacteriochlorins at different positions of the tetrapyrrole ring. The functional consequence of each modification was determined by quantifying the uptake and subcellular deposition of the erlotinib conjugates, cellular response to therapeutic light treatment in tissue cultures, and in eliminating of corresponding tumors grown as a xenograft in SCID mice. The experimental human cancer models the established cell lines UMUC3 (bladder), FaDu (hypopharynx), and primary cultures of head and neck tumor cells. The effectiveness of the compounds was compared to that of HPPH. Furthermore, specific functional contribution of the carboxylic acid side group at position 172 and the chiral methyl group at 3(1') to the overall activity of the chimeric compounds was assessed. Among the conjugates investigated, the PS 10 was identified as the most effective candidate for achieving tumor cell-specific accumulation and yielding improved long-term tumor control.
Figures





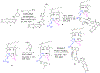
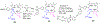

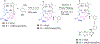


Similar articles
-
Tumor Cell-Specific Retention and Photodynamic Action of Erlotinib-Pyropheophorbide Conjugates.Int J Mol Sci. 2022 Sep 21;23(19):11081. doi: 10.3390/ijms231911081. Int J Mol Sci. 2022. PMID: 36232384 Free PMC article.
-
Epidermal Growth Factor Receptor-Targeted Multifunctional Photosensitizers for Bladder Cancer Imaging and Photodynamic Therapy.J Med Chem. 2019 Mar 14;62(5):2598-2617. doi: 10.1021/acs.jmedchem.8b01927. Epub 2019 Mar 5. J Med Chem. 2019. PMID: 30776232 Free PMC article.
-
Synthesis of novel long wavelength cationic chlorins via stereoselective aldol-like condensation.Bioorg Med Chem Lett. 2012 Mar 1;22(5):1846-9. doi: 10.1016/j.bmcl.2012.01.088. Epub 2012 Jan 31. Bioorg Med Chem Lett. 2012. PMID: 22335896
-
Conjugates of Tetrapyrrolic Macrocycles as Potential Anticancer Target-Oriented Photosensitizers.Top Curr Chem (Cham). 2023 Feb 24;381(2):10. doi: 10.1007/s41061-023-00421-0. Top Curr Chem (Cham). 2023. PMID: 36826755 Review.
-
Bacteriochlorophyll a, and its derivatives: chemistry and perspectives for cancer therapy.Anticancer Agents Med Chem. 2008 Aug;8(6):683-97. Anticancer Agents Med Chem. 2008. PMID: 18690829 Review.
Cited by
-
Molecular engineering of AIE-active boron clustoluminogens for enhanced boron neutron capture therapy.Chem Sci. 2024 Feb 1;15(11):4019-4030. doi: 10.1039/d3sc06222h. eCollection 2024 Mar 13. Chem Sci. 2024. PMID: 38487248 Free PMC article.
-
Tumor Cell-Specific Retention and Photodynamic Action of Erlotinib-Pyropheophorbide Conjugates.Int J Mol Sci. 2022 Sep 21;23(19):11081. doi: 10.3390/ijms231911081. Int J Mol Sci. 2022. PMID: 36232384 Free PMC article.
-
Design Principles and Applications of Fluorescent Kinase Inhibitors for Simultaneous Cancer Bioimaging and Therapy.Cancers (Basel). 2024 Oct 30;16(21):3667. doi: 10.3390/cancers16213667. Cancers (Basel). 2024. PMID: 39518106 Free PMC article. Review.
-
Impact of mono- and di-β-galactose moieties in in vitro / in vivo anticancer efficacy of pyropheophorbide-carbohydrate conjugates by photodynamic therapy.Eur J Med Chem Rep. 2022 Aug;5:100047. doi: 10.1016/j.ejmcr.2022.100047. Epub 2022 Apr 18. Eur J Med Chem Rep. 2022. PMID: 36568335 Free PMC article.
-
Molecular Targeting of Epidermal Growth Factor Receptor (EGFR) and Vascular Endothelial Growth Factor Receptor (VEGFR).Molecules. 2021 Feb 18;26(4):1076. doi: 10.3390/molecules26041076. Molecules. 2021. PMID: 33670650 Free PMC article. Review.
References
-
- Juarranz A; Jaen P; Sanz-Rodriguez F; Cuevas J and Goncalez S Photodynamic therapy of cancer: Basic principles and applications, Clinical and Translational Oncology, 2008, 10, 148–154. - PubMed
-
- Hiramatsu R; Kawabata S; Tanaka H; Sakurai Y; Suzuki M; Ono K; Miyatake S-I; Kuroiwa T; Hao E and Vicente MGH, Tetrakis(p-carboranylthio-tetrafluorophenyl) chlorin (TPFC): Application for photodynamic therapy and boron neutron capture therapy. J. Pharmaceutical Sci, 2015, 104, 962–970 - PubMed
-
- Lo P-C; Rodriguez-Morgade MS; Pandey RK; Ng DKP; Torres T and Dumoulin F The unique features and promises of phthalocyanines as advanced photosensitizers for photodynamic therapy of cancer. Chem. Soc. Rev, 2020, 49, 1041–1056. - PubMed
-
- Shaker YM; Sweed AMK; Moylan C; Rogers L; Ryan AA; Pettidemange R and Senge MO, Current developments in using MESO-(TETRA) substituted porphyrins in PDT in HANDBOOK OF PHOTODYNAMIC THERAPY, Updates on Recent Applications of Porphyrin-Based Compound (Eds: Pandey RK; Kessel D and Dougherty TJ), World Scientific, New Jersey, 2016, pp 95–149.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

