Estimation of US SARS-CoV-2 Infections, Symptomatic Infections, Hospitalizations, and Deaths Using Seroprevalence Surveys
- PMID: 33399860
- PMCID: PMC7786245
- DOI: 10.1001/jamanetworkopen.2020.33706
Estimation of US SARS-CoV-2 Infections, Symptomatic Infections, Hospitalizations, and Deaths Using Seroprevalence Surveys
Abstract
Importance: Estimates of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease burden are needed to help guide interventions.
Objective: To estimate the number of SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths in the US as of November 15, 2020.
Design, setting, and participants: In this cross-sectional study of respondents of all ages, data from 4 regional and 1 nationwide Centers for Disease Control and Prevention (CDC) seroprevalence surveys (April [n = 16 596], May, June, and July [n = 40 817], and August [n = 38 355]) were used to estimate infection underreporting multipliers and symptomatic underreporting multipliers. Community serosurvey data from randomly selected members of the general population were also used to validate the underreporting multipliers.
Main outcomes and measures: SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths. The median of underreporting multipliers derived from the 5 CDC seroprevalence surveys in the 10 states that participated in 2 or more surveys were applied to surveillance data of reported coronavirus disease 2019 (COVID-19) cases for 5 respective time periods to derive estimates of SARS-CoV-2 infections and symptomatic infections, which were summed to estimate SARS-CoV-2 infections and symptomatic infections in the US. Estimates of infections and symptomatic infections were combined with estimates of the hospitalization ratio and fatality ratio to derive estimates of SARS-CoV-2 hospitalizations and deaths. External validity of the surveys was evaluated with the April CDC survey by comparing results to 5 serosurveys (n = 22 118) that used random sampling of the general population. Internal validity of the multipliers from the 10 specific states was assessed in the August CDC survey by comparing multipliers from the 10 states to all states. A sensitivity analysis was conducted using the interquartile range of the multipliers to derive a high and low estimate of SARS-CoV-2 infections and symptomatic infections. The underreporting multipliers were then used to adjust the reported COVID-19 infections to estimate the full SARS-COV-2 disease burden.
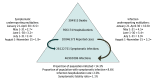
Results: Adjusting reported COVID-19 infections using underreporting multipliers derived from CDC seroprevalence studies in April (n = 16 596), May (n = 14 291), June (n = 14 159), July (n = 12 367), and August (n = 38 355), there were estimated medians of 46 910 006 (interquartile range [IQR], 38 192 705-60 814 748) SARS-CoV-2 infections, 28 122 752 (IQR, 23 014 957-36 438 592) symptomatic infections, 956 174 (IQR, 782 509-1 238 912) hospitalizations, and 304 915 (IQR, 248 253-395 296) deaths in the US through November 15, 2020. An estimated 14.3% (IQR, 11.6%-18.5%) of the US population were infected by SARS-CoV-2 as of mid-November 2020.
Conclusions and relevance: The SARS-CoV-2 disease burden may be much larger than reported COVID-19 cases owing to underreporting. Even after adjusting for underreporting, a substantial gap remains between the estimated proportion of the population infected and the proportion infected required to reach herd immunity. Additional seroprevalence surveys are needed to monitor the pandemic, including after the introduction of safe and efficacious vaccines.
Conflict of interest statement
Figures
Similar articles
-
Estimated SARS-CoV-2 Seroprevalence in the US as of September 2020.JAMA Intern Med. 2021 Apr 1;181(4):450-460. doi: 10.1001/jamainternmed.2020.7976. JAMA Intern Med. 2021. PMID: 33231628 Free PMC article.
-
Estimated US Infection- and Vaccine-Induced SARS-CoV-2 Seroprevalence Based on Blood Donations, July 2020-May 2021.JAMA. 2021 Oct 12;326(14):1400-1409. doi: 10.1001/jama.2021.15161. JAMA. 2021. PMID: 34473201 Free PMC article.
-
SARS-CoV-2 Infection Hospitalization Rate and Infection Fatality Rate Among the Non-Congregate Population in Connecticut.Am J Med. 2021 Jun;134(6):812-816.e2. doi: 10.1016/j.amjmed.2021.01.020. Epub 2021 Feb 20. Am J Med. 2021. PMID: 33617808 Free PMC article.
-
Systematic literature review of SARS-CoV-2 seroprevalence surveys in Canada through April 2021.IJID Reg. 2022 Sep;4:157-164. doi: 10.1016/j.ijregi.2022.07.010. Epub 2022 Jul 29. IJID Reg. 2022. PMID: 35919829 Free PMC article. Review.
-
Population-based seroprevalence surveys of anti-SARS-CoV-2 antibody: An up-to-date review.Int J Infect Dis. 2020 Dec;101:314-322. doi: 10.1016/j.ijid.2020.10.011. Epub 2020 Oct 9. Int J Infect Dis. 2020. PMID: 33045429 Free PMC article. Review.
Cited by
-
Clinical and Economic Effects of Widespread Rapid Testing to Decrease SARS-CoV-2 Transmission.Ann Intern Med. 2021 Jun;174(6):803-810. doi: 10.7326/M21-0510. Epub 2021 Mar 9. Ann Intern Med. 2021. PMID: 33683930 Free PMC article.
-
A simple SEIR-V model to estimate COVID-19 prevalence and predict SARS-CoV-2 transmission using wastewater-based surveillance data.medRxiv [Preprint]. 2022 Jul 18:2022.07.17.22277721. doi: 10.1101/2022.07.17.22277721. medRxiv. 2022. Update in: Sci Total Environ. 2023 Jan 20;857(Pt 1):159326. doi: 10.1016/j.scitotenv.2022.159326 PMID: 35898336 Free PMC article. Updated. Preprint.
-
Underreporting of Cases in the COVID-19 Outbreak of Borriana (Spain) during Mass Gathering Events in March 2020: A Cross-Sectional Study.Epidemiologia (Basel). 2024 Aug 9;5(3):499-510. doi: 10.3390/epidemiologia5030034. Epidemiologia (Basel). 2024. PMID: 39189253 Free PMC article.
-
Seroprevalence of Severe Acute Respiratory Syndrome Coronavirus 2 After the Second Wave in South Africa in Human Immunodeficiency Virus-Infected and Uninfected Persons: A Cross-Sectional Household Survey.Clin Infect Dis. 2022 Aug 24;75(1):e57-e68. doi: 10.1093/cid/ciac198. Clin Infect Dis. 2022. PMID: 35271693 Free PMC article.
-
Ecological studies of COVID-19 and air pollution: How useful are they?Environ Epidemiol. 2022 Feb 4;6(1):e195. doi: 10.1097/EE9.0000000000000195. eCollection 2022 Feb. Environ Epidemiol. 2022. PMID: 35169673 Free PMC article.
References
-
- Centers for Disease Control and Prevention . Cases in the US. Accessed November 12, 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
-
- Krantz SG, Rao ASRS. Level of underreporting including underdiagnosis before the first peak of COVID-19 in various countries: preliminary retrospective results based on wavelets and deterministic modeling. Infect Control Hosp Epidemiol. 2020;41(7):857-859. doi:10.1017/ice.2020.116 - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention . COVID-19 pandemic planning scenarios. Updated July 1, 2020. Accessed November 12, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios-h.pdf
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous