Roles of the HOX Proteins in Cancer Invasion and Metastasis
- PMID: 33375038
- PMCID: PMC7792759
- DOI: 10.3390/cancers13010010
Roles of the HOX Proteins in Cancer Invasion and Metastasis
Abstract
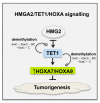
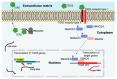
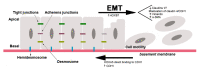
Invasion and metastasis correspond to the foremost cause of cancer-related death, and the molecular networks behind these two processes are extremely complex and dependent on the intra- and extracellular conditions along with the prime of the premetastatic niche. Currently, several studies suggest an association between the levels of HOX genes expression and cancer cell invasion and metastasis, which favour the formation of novel tumour masses. The deregulation of HOX genes by HMGA2/TET1 signalling and the regulatory effect of noncoding RNAs generated by the HOX loci can also promote invasion and metastasis, interfering with the expression of HOX genes or other genes relevant to these processes. In this review, we present five molecular mechanisms of HOX deregulation by which the HOX clusters products may affect invasion and metastatic processes in solid tumours.
Keywords: HMGA2/TET1/HOXA signalling pathway; HOX; TGFβ signalling; epithelial-to-mesenchymal transition; invasion and metastasis; lncRNAs; microRNAs.
Conflict of interest statement
Authors declare there is no conflict of interests.
Figures

Similar articles
-
In silico interaction of HOX cluster-embedded microRNAs and long non-coding RNAs in oral cancer.J Oral Pathol Med. 2022 Jan;51(1):18-29. doi: 10.1111/jop.13225. Epub 2021 Aug 16. J Oral Pathol Med. 2022. PMID: 34358375 Review.
-
The regulatory role of HOX interacting lncRNA in oral cancer-An in silico analysis.J Oral Pathol Med. 2022 Sep;51(8):684-693. doi: 10.1111/jop.13329. Epub 2022 Aug 7. J Oral Pathol Med. 2022. PMID: 35766359 Review.
-
HOX cluster-embedded antisense long non-coding RNAs in lung cancer.Cancer Lett. 2019 May 28;450:14-21. doi: 10.1016/j.canlet.2019.02.036. Epub 2019 Feb 23. Cancer Lett. 2019. PMID: 30807784 Review.
-
HOX cluster-embedded lncRNAs and epithelial-mesenchymal transition in cancer: Molecular mechanisms and therapeutic opportunities.Biochim Biophys Acta Rev Cancer. 2023 Jan;1878(1):188840. doi: 10.1016/j.bbcan.2022.188840. Epub 2022 Nov 17. Biochim Biophys Acta Rev Cancer. 2023. PMID: 36403923 Review.
-
Multiple roles of HOX proteins in Metastasis: Let me count the ways.Cancer Metastasis Rev. 2020 Sep;39(3):661-679. doi: 10.1007/s10555-020-09908-4. Cancer Metastasis Rev. 2020. PMID: 32572656 Review.
Cited by
-
Homeobox Genes in Cancers: From Carcinogenesis to Recent Therapeutic Intervention.Front Oncol. 2021 Oct 14;11:770428. doi: 10.3389/fonc.2021.770428. eCollection 2021. Front Oncol. 2021. PMID: 34722321 Free PMC article. Review.
-
Effects of MLL5 and HOXA regulated by NRP1 on radioresistance in A549.Oncol Lett. 2021 May;21(5):403. doi: 10.3892/ol.2021.12664. Epub 2021 Mar 19. Oncol Lett. 2021. PMID: 33777226 Free PMC article.
-
PBX4 functions as a potential novel oncopromoter in colorectal cancer: a comprehensive analysis of the PBX gene family.Am J Cancer Res. 2022 Feb 15;12(2):585-600. eCollection 2022. Am J Cancer Res. 2022. PMID: 35261789 Free PMC article.
-
HOXD1 inhibits lung adenocarcinoma progression and is regulated by DNA methylation.Oncol Rep. 2024 Dec;52(6):173. doi: 10.3892/or.2024.8832. Epub 2024 Oct 25. Oncol Rep. 2024. PMID: 39450540 Free PMC article.
-
S1PR1 induces metabolic reprogramming of ceramide in vascular endothelial cells, affecting hepatocellular carcinoma angiogenesis and progression.Cell Death Dis. 2022 Sep 6;13(9):768. doi: 10.1038/s41419-022-05210-z. Cell Death Dis. 2022. PMID: 36068200 Free PMC article.
References
-
- Damrauer J.S., Phelps S.N., Amuchastegui K., Lupo R., Mabe N.W., Walens A., Kroger B.R., Alvarez J.V. Foxo-dependent Par-4 Upregulation Prevents Long-term Survival of Residual Cells Following PI3K-Akt Inhibition. Mol. Cancer Res. Mcr. 2018;16:599–609. doi: 10.1158/1541-7786.MCR-17-0492. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

