Inhibition of Exo-miR-19a-3p derived from cardiomyocytes promotes angiogenesis and improves heart function in mice with myocardial infarction via targeting HIF-1α
- PMID: 33352533
- PMCID: PMC7762502
- DOI: 10.18632/aging.103563
Inhibition of Exo-miR-19a-3p derived from cardiomyocytes promotes angiogenesis and improves heart function in mice with myocardial infarction via targeting HIF-1α
Abstract
Background: Myocardial infarction (MI), a common presentation for cardiovascular disease, is caused by reduction of blood flow and oxygen supply and is one of the main causes of death worldwide. MicroRNAs participate in multiple physiological and pathological processed and play crucial role in myocardial infarction.
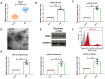
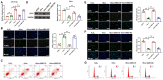
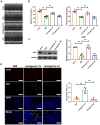
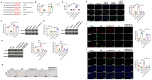
Results: qRT-PCR analysis showed that expression level of miR-19a-3p was increased in serum of patient with MI. In vitro study indicated that the miR-19a-3p level was upregulated in response to H2O2 treatment and transferred by exosome, and then, uptake occurred in endothelial cells. Furthermore, western blot and immunostaining showed that treatment of exosome enriched miR-19a-3p suppressed the proliferation of endothelial cells and induced cell death, which was inhibited by AMO-19 transfection. Administration of antagomiR-19a-3p promoted angiogenesis and improved heart function of MI mice. Moreover, miR-19a-3p overexpression downregulated the protein level of HIF-1α and transfection of si-HIF-1α reversed the promotion of endothelial cells proliferation caused by AMO-19 transfection. In addition, antagomiR-19a-3p treatment accelerated angiogenesis and infection of AAV5-shHIF-1α inhibited that effect in MI mice.
Conclusions: In conclusion, our finding indicated that miR-19a-3p inhibited endothelial cells proliferation and angiogenesis via targeting HIF-1α and attenuated heart function of mice after MI, and suggested a new mechanism of cell-to-cell communication between cardiomyocytes and endothelial cells.
Keywords: HIF-1α; angiogenesis; exosome; miR-19a-3p; myocardial infarction.
Conflict of interest statement
Figures
Similar articles
-
Astragaloside IV-induced BMSC exosomes promote neovascularization and protect cardiac function in myocardial infarction mice via the miR-411/HIF-1α axis.J Liposome Res. 2024 Sep;34(3):452-463. doi: 10.1080/08982104.2023.2293844. Epub 2023 Dec 25. J Liposome Res. 2024. PMID: 38088046
-
Blocking exosomal miRNA-153-3p derived from bone marrow mesenchymal stem cells ameliorates hypoxia-induced myocardial and microvascular damage by targeting the ANGPT1-mediated VEGF/PI3k/Akt/eNOS pathway.Cell Signal. 2021 Jan;77:109812. doi: 10.1016/j.cellsig.2020.109812. Epub 2020 Oct 24. Cell Signal. 2021. PMID: 33164880
-
MiR-221-3p targets Hif-1α to inhibit angiogenesis in heart failure.Lab Invest. 2021 Jan;101(1):104-115. doi: 10.1038/s41374-020-0450-3. Epub 2020 Sep 1. Lab Invest. 2021. PMID: 32873879
-
Hypoxia-inducible factor-1: Regulatory mechanisms and drug therapy in myocardial infarction.Eur J Pharmacol. 2024 Jan 15;963:176277. doi: 10.1016/j.ejphar.2023.176277. Epub 2023 Dec 18. Eur J Pharmacol. 2024. PMID: 38123007 Review.
-
Reviewing the role of cardiac exosomes in myocardial repair at a glance.Cell Biol Int. 2021 Jul;45(7):1352-1363. doi: 10.1002/cbin.11515. Epub 2021 Apr 13. Cell Biol Int. 2021. PMID: 33289229 Review.
Cited by
-
Regulation mechanism of microRNAs in cardiac cells-derived exosomes in cell crosstalk.Front Pharmacol. 2024 Aug 20;15:1399850. doi: 10.3389/fphar.2024.1399850. eCollection 2024. Front Pharmacol. 2024. PMID: 39228519 Free PMC article. Review.
-
Retraction Note: Mesenchymal stem cell-derived exosome miR-542-3p suppresses inflammation and prevents cerebral infarction.Stem Cell Res Ther. 2023 Jan 16;14(1):9. doi: 10.1186/s13287-022-03227-x. Stem Cell Res Ther. 2023. PMID: 36647168 Free PMC article. No abstract available.
-
MSCs-Derived Extracellular Vesicles Carrying miR-212-5p Alleviate Myocardial Infarction-Induced Cardiac Fibrosis via NLRC5/VEGF/TGF-β1/SMAD Axis.J Cardiovasc Transl Res. 2022 Apr;15(2):302-316. doi: 10.1007/s12265-021-10156-2. Epub 2021 Sep 10. J Cardiovasc Transl Res. 2022. PMID: 34508321
-
Allicin protects against myocardial I/R by accelerating angiogenesis via the miR-19a-3p/PI3K/AKT axis.Aging (Albany NY). 2021 Oct 4;13(19):22843-22855. doi: 10.18632/aging.203578. Epub 2021 Oct 4. Aging (Albany NY). 2021. PMID: 34607973 Free PMC article.
-
Emerging roles of the RNA modifications N6-methyladenosine and adenosine-to-inosine in cardiovascular diseases.Mol Ther Nucleic Acids. 2022 Jul 20;29:426-461. doi: 10.1016/j.omtn.2022.07.018. eCollection 2022 Sep 13. Mol Ther Nucleic Acids. 2022. PMID: 35991314 Free PMC article. Review.
References
-
- Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guérin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, Dumeau E, Kotti S, Bruneval P, et al.. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013; 19:1273–80. 10.1038/nm.3284 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

