Neutrophils and Macrophages as Targets for Development of Nanotherapeutics in Inflammatory Diseases
- PMID: 33348630
- PMCID: PMC7766591
- DOI: 10.3390/pharmaceutics12121222
Neutrophils and Macrophages as Targets for Development of Nanotherapeutics in Inflammatory Diseases
Abstract
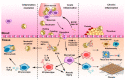
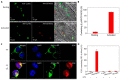
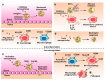
Neutrophils and macrophages are major components of innate systems, playing central roles in inflammation responses to infections and tissue injury. If they are out of control, inflammation responses can cause the pathogenesis of a wide range of diseases, such as inflammatory disorders and autoimmune diseases. Precisely regulating the functions of neutrophils and macrophages in vivo is a potential strategy to develop immunotherapies to treat inflammatory diseases. Advances in nanotechnology have enabled us to design nanoparticles capable of targeting neutrophils or macrophages in vivo. This review discusses the current status of how nanoparticles specifically target neutrophils or macrophages and how they manipulate leukocyte functions to inhibit their activation for inflammation resolution or to restore their defense ability for pathogen clearance. Finally, we present a novel concept of hijacking leukocytes to deliver nanotherapeutics across the blood vessel barrier. This review highlights the challenges and opportunities in developing nanotherapeutics to target leukocytes for improved treatment of inflammatory diseases.
Keywords: cell targeting; inflammatory diseases; interactions of nanoparticles and cells; macrophages; nanomedicine; neutrophils.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Neutrophil-mediated delivery of nanotherapeutics across blood vessel barrier.Ther Deliv. 2018 Jan;9(1):29-35. doi: 10.4155/tde-2017-0081. Ther Deliv. 2018. PMID: 29216803 Free PMC article.
-
Drug nanocarriers to treat autoimmunity and chronic inflammatory diseases.Semin Immunol. 2017 Dec;34:61-67. doi: 10.1016/j.smim.2017.08.010. Epub 2017 Aug 30. Semin Immunol. 2017. PMID: 28855088 Free PMC article. Review.
-
Neutrophil-Based Delivery Systems for Nanotherapeutics.Small. 2018 Oct;14(42):e1801674. doi: 10.1002/smll.201801674. Epub 2018 Aug 24. Small. 2018. PMID: 30144279 Review.
-
Neutrophil-Based Drug Delivery Systems.Adv Mater. 2018 May;30(22):e1706245. doi: 10.1002/adma.201706245. Epub 2018 Mar 26. Adv Mater. 2018. PMID: 29577477 Free PMC article. Review.
-
Leukocyte-derived biomimetic nanoparticulate drug delivery systems for cancer therapy.Acta Pharm Sin B. 2018 Jan;8(1):4-13. doi: 10.1016/j.apsb.2017.12.001. Epub 2018 Jan 10. Acta Pharm Sin B. 2018. PMID: 29872618 Free PMC article. Review.
Cited by
-
Pycnogenol-Assisted Alleviation of Titanium Dioxide Nanoparticle-Induced Lung Inflammation via Thioredoxin-Interacting Protein Downregulation.Antioxidants (Basel). 2024 Aug 9;13(8):972. doi: 10.3390/antiox13080972. Antioxidants (Basel). 2024. PMID: 39199218 Free PMC article.
-
Immunostimulation Signaling via Toll-like Receptor 2 Activation: A Molecular Mechanism of Lactococcus lactis OTG1204 In Vitro and In Vivo.Nutrients. 2024 Oct 25;16(21):3629. doi: 10.3390/nu16213629. Nutrients. 2024. PMID: 39519462 Free PMC article.
-
THP-1 Macrophages Limit Neutrophil Transendothelial Migration in a Model Infection.Cell Mol Bioeng. 2024 Jul 20;17(4):279-293. doi: 10.1007/s12195-024-00813-2. eCollection 2024 Aug. Cell Mol Bioeng. 2024. PMID: 39372553
-
Consequence of alcohol intoxication-mediated efferocytosis impairment.Front Immunol. 2024 Jul 22;15:1386658. doi: 10.3389/fimmu.2024.1386658. eCollection 2024. Front Immunol. 2024. PMID: 39104537 Free PMC article. Review.
-
Targeted nanoparticles modify neutrophil function in vivo.Front Immunol. 2022 Oct 5;13:1003871. doi: 10.3389/fimmu.2022.1003871. eCollection 2022. Front Immunol. 2022. PMID: 36275643 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

