Function of the endolysosomal network in cholesterol homeostasis and metabolic-associated fatty liver disease (MAFLD)
- PMID: 33348067
- PMCID: PMC8324686
- DOI: 10.1016/j.molmet.2020.101146
Function of the endolysosomal network in cholesterol homeostasis and metabolic-associated fatty liver disease (MAFLD)
Abstract
Background: Metabolic-associated fatty liver disease (MAFLD), also known as non-alcoholic fatty liver disease, has become the leading cause of chronic liver disease worldwide. In addition to hepatic accumulation of triglycerides, dysregulated cholesterol metabolism is an important contributor to the pathogenesis of MAFLD. Maintenance of cholesterol homeostasis is highly dependent on cellular cholesterol uptake and, subsequently, cholesterol transport to other membrane compartments, such as the endoplasmic reticulum (ER).
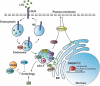
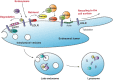
Scope of review: The endolysosomal network is key for regulating cellular homeostasis and adaptation, and emerging evidence has shown that the endolysosomal network is crucial to maintain metabolic homeostasis. In this review, we will summarize our current understanding of the role of the endolysosomal network in cholesterol homeostasis and its implications in MAFLD pathogenesis.
Major conclusions: Although multiple endolysosomal proteins have been identified in the regulation of cholesterol uptake, intracellular transport, and degradation, their physiological role is incompletely understood. Further research should elucidate their role in controlling metabolic homeostasis and development of fatty liver disease.
Keywords: Cholesterol transport; Endosomal sorting; Endosome and lysosome; LDLR; NAFLD; PCSK9.
Copyright © 2020 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures
Similar articles
-
Metabolic-associated fatty liver disease and lipoprotein metabolism.Mol Metab. 2021 Aug;50:101238. doi: 10.1016/j.molmet.2021.101238. Epub 2021 Apr 20. Mol Metab. 2021. PMID: 33892169 Free PMC article. Review.
-
Polyribonucleotide nucleotidyltransferase 1 participates in metabolic-associated fatty liver disease pathogenesis by affecting lipid metabolism and mitochondrial homeostasis.Mol Metab. 2024 Nov;89:102022. doi: 10.1016/j.molmet.2024.102022. Epub 2024 Aug 31. Mol Metab. 2024. PMID: 39218215 Free PMC article.
-
Loss and gain of function of Grp75 or mitofusin 2 distinctly alter cholesterol metabolism, but all promote triglyceride accumulation in hepatocytes.Biochim Biophys Acta Mol Cell Biol Lipids. 2021 Dec;1866(12):159030. doi: 10.1016/j.bbalip.2021.159030. Epub 2021 Aug 20. Biochim Biophys Acta Mol Cell Biol Lipids. 2021. PMID: 34419589
-
Neutrophil: An emerging player in the occurrence and progression of metabolic associated fatty liver disease.Int Immunopharmacol. 2021 Aug;97:107609. doi: 10.1016/j.intimp.2021.107609. Epub 2021 Apr 19. Int Immunopharmacol. 2021. PMID: 33887577 Review.
-
An optical nanoreporter of endolysosomal lipid accumulation reveals enduring effects of diet on hepatic macrophages in vivo.Sci Transl Med. 2018 Oct 3;10(461):eaar2680. doi: 10.1126/scitranslmed.aar2680. Sci Transl Med. 2018. PMID: 30282694 Free PMC article.
Cited by
-
Metabolic-associated fatty liver disease and lipoprotein metabolism.Mol Metab. 2021 Aug;50:101238. doi: 10.1016/j.molmet.2021.101238. Epub 2021 Apr 20. Mol Metab. 2021. PMID: 33892169 Free PMC article. Review.
-
Inhibition of Hepatic AMPK Pathway Contributes to Free Fatty Acids-Induced Fatty Liver Disease in Laying Hen.Metabolites. 2022 Sep 1;12(9):825. doi: 10.3390/metabo12090825. Metabolites. 2022. PMID: 36144229 Free PMC article.
-
High-density lipoprotein cholesterol trajectory and new-onset metabolic dysfunction-associated fatty liver disease incidence: a longitudinal study.Diabetol Metab Syndr. 2024 Sep 11;16(1):223. doi: 10.1186/s13098-024-01457-y. Diabetol Metab Syndr. 2024. PMID: 39261925 Free PMC article.
-
Pathways and Mechanisms of Cellular Cholesterol Efflux-Insight From Imaging.Front Cell Dev Biol. 2022 Mar 1;10:834408. doi: 10.3389/fcell.2022.834408. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35300409 Free PMC article. Review.
-
Alteration in Rab11-mediated endocytic trafficking of LDL receptor contributes to angiotensin II-induced cholesterol accumulation and injury in podocytes.Cell Prolif. 2022 Jun;55(6):e13229. doi: 10.1111/cpr.13229. Epub 2022 May 14. Cell Prolif. 2022. PMID: 35567428 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

