Sulfonimide-Based Dendrimers: Progress in Synthesis, Characterization, and Potential Applications
- PMID: 33333758
- PMCID: PMC7765173
- DOI: 10.3390/polym12122987
Sulfonimide-Based Dendrimers: Progress in Synthesis, Characterization, and Potential Applications
Abstract
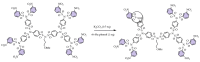
There are more than 50 families of dendrimers, and some of which, such as polyamidoamine PAMAM, are well studied, and some are just starting to attract the attention of researchers. One promising type of dendrimers is sulfonimide-based dendrimers (SBDs). To date, SBDs are used in organic synthesis as starting reagents for the convergent synthesis of higher generations dendrimers, in materials science as alternative electrolyte solutions for fuel cells, and in medicinal chemistry as potential substances for drug transfer procedures. Despite the fact that most dendrimers are amorphous substances among the SBDs, several structures are distinguished that are prone to the formation of crystalline solids with melting points in the range of 120-250 °C. Similar to those of other dendrimers, the chemical and physical properties of SBDs depend on their outer shell, which is formed by functional groups. To date, SBDs decorated with end groups such as naphthyl, nitro, methyl, and methoxy have been successfully synthesized, and each of these groups gives the dendrimers specific properties. Analysis of the structure of SBD, their synthesis methods, and applications currently available in the literature reveals that these dendrimers have not yet been fully explored.
Keywords: dendrimers; dendritic polymers; dendrons; divergent approach; functionalization; generations; sulfonimide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Designer dendrimers: branched oligosulfonimides with controllable molecular architectures.J Am Chem Soc. 2006 Jul 12;128(27):8964-74. doi: 10.1021/ja061606b. J Am Chem Soc. 2006. PMID: 16819893
-
Synthesis of symmetrical and unsymmetrical PAMAM dendrimers by fusion between azide- and alkyne-functionalized PAMAM dendrons.Bioconjug Chem. 2007 Mar-Apr;18(2):579-84. doi: 10.1021/bc060256f. Epub 2007 Mar 3. Bioconjug Chem. 2007. PMID: 17335177
-
Planning Implications Related to Sterilization-Sensitive Science Investigations Associated with Mars Sample Return (MSR).Astrobiology. 2022 Jun;22(S1):S112-S164. doi: 10.1089/AST.2021.0113. Epub 2022 May 19. Astrobiology. 2022. PMID: 34904892
-
Chemical Structure and Surface Modification of Dendritic Nanomaterials Tailored for Therapeutic and Diagnostic Applications.Curr Top Med Chem. 2017;17(13):1542-1554. doi: 10.2174/1568026616666161222104112. Curr Top Med Chem. 2017. PMID: 28017148 Review.
-
Click dendrimers and triazole-related aspects: catalysts, mechanism, synthesis, and functions. A bridge between dendritic architectures and nanomaterials.Acc Chem Res. 2012 Apr 17;45(4):630-40. doi: 10.1021/ar200235m. Epub 2011 Dec 8. Acc Chem Res. 2012. PMID: 22148925 Review.
References
-
- Ratner B.D., Hoffman A.S., Schoen F.J., Lemons J.E. Biomaterials Science. Elsevier; Amsterdam, The Netherlands: 2013.
-
- McCarthy T.D., Karellas P., Henderson S.A., Giannis M., O’Keefe D.F., Heery G., Paull J.R.A., Matthews B.R., Holan G. Dendrimers as drugs: Discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol. Pharm. 2005;2:312–318. doi: 10.1021/mp050023q. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

