Monitoring of the Complement System Status in Patients With B-Cell Malignancies Treated With Rituximab
- PMID: 33329558
- PMCID: PMC7710700
- DOI: 10.3389/fimmu.2020.584509
Monitoring of the Complement System Status in Patients With B-Cell Malignancies Treated With Rituximab
Abstract
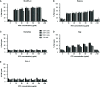
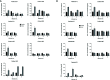
Rituximab is a pioneering anti-CD20 monoclonal antibody that became the first-line drug used in immunotherapy of B-cell malignancies over the last twenty years. Rituximab activates the complement system in vitro, but there is an ongoing debate on the exact role of this effector mechanism in therapeutic effect. Results of both in vitro and in vivo studies are model-dependent and preclude clear clinical conclusions. Additional confounding factors like complement inhibition by tumor cells, loss of target antigen and complement depletion due to excessively applied immunotherapeutics, intrapersonal variability in the concentration of main complement components and differences in tumor burden all suggest that a personalized approach is the best strategy for optimization of rituximab dosage and therapeutic schedule. Herein we critically review the existing knowledge in support of such concept and present original data on markers of complement activation, complement consumption, and rituximab accumulation in plasma of patients with chronic lymphocytic leukemia (CLL) and non-Hodgkin's lymphomas (NHL). The increase of markers such as C4d and terminal complement complex (TCC) suggest the strongest complement activation after the first administration of rituximab, but not indicative of clinical outcome in patients receiving rituximab in combination with chemotherapy. Both ELISA and complement-dependent cytotoxicity (CDC) functional assay showed that a substantial number of patients accumulate rituximab to the extent that consecutive infusions do not improve the cytotoxic capacity of their sera. Our data suggest that individual assessment of CDC activity and rituximab concentration in plasma may support clinicians' decisions on further drug infusions, or instead prescribing a therapy with anti-CD20 antibodies like obinutuzumab that more efficiently activate effector mechanisms other than complement.
Keywords: chronic lymphocytic leukemia; complement system; non-Hodgkin’s lymphoma; obinutuzumab (GA101); rituximab.
Copyright © 2020 Felberg, Taszner, Urban, Majeranowski, Jaskuła, Jurkiewicz, Stasiłojć, Blom, Zaucha and Okrój.
Figures


Similar articles
-
Exhaustion of cytotoxic effector systems may limit monoclonal antibody-based immunotherapy in cancer patients.J Immunol. 2012 Apr 1;188(7):3532-41. doi: 10.4049/jimmunol.1103693. Epub 2012 Feb 24. J Immunol. 2012. PMID: 22368276 Free PMC article. Clinical Trial.
-
Role for ZAP-70 Signaling in the Differential Effector Functions of Rituximab and Obinutuzumab (GA101) in Chronic Lymphocytic Leukemia B Cells.J Immunol. 2017 Aug 15;199(4):1275-1282. doi: 10.4049/jimmunol.1602105. Epub 2017 Jul 14. J Immunol. 2017. PMID: 28710251
-
Complement-Dependent Activity of CD20-Specific IgG Correlates With Bivalent Antigen Binding and C1q Binding Strength.Front Immunol. 2021 Jan 11;11:609941. doi: 10.3389/fimmu.2020.609941. eCollection 2020. Front Immunol. 2021. PMID: 33505398 Free PMC article.
-
A Review of Obinutuzumab (GA101), a Novel Type II Anti-CD20 Monoclonal Antibody, for the Treatment of Patients with B-Cell Malignancies.Adv Ther. 2017 Feb;34(2):324-356. doi: 10.1007/s12325-016-0451-1. Epub 2016 Dec 21. Adv Ther. 2017. PMID: 28004361 Free PMC article. Review.
-
Obinutuzumab for B-cell malignancies.Expert Opin Biol Ther. 2014 Aug;14(8):1197-205. doi: 10.1517/14712598.2014.922535. Epub 2014 May 23. Expert Opin Biol Ther. 2014. PMID: 24856933 Review.
Cited by
-
An anti-HER2 biparatopic antibody that induces unique HER2 clustering and complement-dependent cytotoxicity.Nat Commun. 2023 Mar 13;14(1):1394. doi: 10.1038/s41467-023-37029-3. Nat Commun. 2023. PMID: 36914633 Free PMC article.
-
Substitutions at position 263 within the von Willebrand factor type A domain determine the functionality of complement C2 protein.Front Immunol. 2022 Dec 15;13:1061696. doi: 10.3389/fimmu.2022.1061696. eCollection 2022. Front Immunol. 2022. PMID: 36591303 Free PMC article.
-
miR-132-3p regulates antibody-mediated complement-dependent cytotoxicity in colon cancer cells by directly targeting CD55.Clin Exp Immunol. 2023 Mar 8;211(1):57-67. doi: 10.1093/cei/uxac120. Clin Exp Immunol. 2023. PMID: 36571232 Free PMC article.
-
Accurate Visualization of C4d Complement Fragment in Immunohistochemistry by C-Terminal Linear Neoepitope-Specific Antibodies.Int J Mol Sci. 2024 Sep 30;25(19):10526. doi: 10.3390/ijms251910526. Int J Mol Sci. 2024. PMID: 39408855 Free PMC article.
-
Overview on the Link Between the Complement System and Auto-Immune Articular and Pulmonary Disease.Open Access Rheumatol. 2023 May 15;15:65-79. doi: 10.2147/OARRR.S318826. eCollection 2023. Open Access Rheumatol. 2023. PMID: 37214353 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

