Lung involvement in monogenic interferonopathies
- PMID: 33328278
- PMCID: PMC9489100
- DOI: 10.1183/16000617.0001-2020
Lung involvement in monogenic interferonopathies
Abstract
Monogenic type I interferonopathies are inherited heterogeneous disorders characterised by early onset of systemic and organ specific inflammation, associated with constitutive activation of type I interferons (IFNs). In the last few years, several clinical reports identified the lung as one of the key target organs of IFN-mediated inflammation. The major pulmonary patterns described comprise children's interstitial lung diseases (including diffuse alveolar haemorrhages) and pulmonary arterial hypertension but diagnosis may be challenging. Respiratory symptoms may be either mild or absent at disease onset and variably associated with systemic or organ specific inflammation. In addition, associated extrapulmonary clinical features may precede lung function impairment by years, and patients may display severe/endstage lung involvement, although this may be clinically hidden during the long-term disease course. Conversely, a few cases of atypical severe lung involvement at onset have been reported without clinically manifested extrapulmonary signs. Hence, a multidisciplinary approach involving pulmonologists, paediatricians and rheumatologists should always be considered when a monogenic interferonopathy is suspected. Pulmonologists should also be aware of the main pattern of presentation to allow prompt diagnosis and a targeted therapeutic strategy. In this regard, promising therapeutic strategies rely on Janus kinase-1/2 (JAK-1/2) inhibitors blocking the type I IFN-mediated intracellular cascade.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: S. Cazzato has nothing to disclose. Conflict of interest: A. Omenetti has nothing to disclose. Conflict of interest: C. Ravaglia has nothing to disclose. Conflict of interest: V. Poletti has nothing to disclose.
Figures
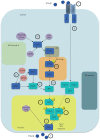
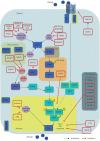

Similar articles
-
Overview of STING-Associated Vasculopathy with Onset in Infancy (SAVI) Among 21 Patients.J Allergy Clin Immunol Pract. 2021 Feb;9(2):803-818.e11. doi: 10.1016/j.jaip.2020.11.007. Epub 2020 Nov 18. J Allergy Clin Immunol Pract. 2021. PMID: 33217613
-
[When to consider type I interferonopathy in adulthood?].Rev Med Interne. 2022 Jun;43(6):347-355. doi: 10.1016/j.revmed.2021.11.003. Epub 2022 Feb 15. Rev Med Interne. 2022. PMID: 35177256 French.
-
Lung Inflammation in STING-Associated Vasculopathy with Onset in Infancy (SAVI).Cells. 2022 Jan 18;11(3):318. doi: 10.3390/cells11030318. Cells. 2022. PMID: 35159128 Free PMC article. Review.
-
JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies.J Clin Invest. 2018 Jul 2;128(7):3041-3052. doi: 10.1172/JCI98814. Epub 2018 Jun 11. J Clin Invest. 2018. PMID: 29649002 Free PMC article.
-
The role of interferons type I, II and III in myositis: A review.Brain Pathol. 2021 May;31(3):e12955. doi: 10.1111/bpa.12955. Brain Pathol. 2021. PMID: 34043262 Free PMC article. Review.
Cited by
-
Type I Interferonopathies in Childhood.Balkan Med J. 2023 May 8;40(3):165-174. doi: 10.4274/balkanmedj.galenos.2023.2023-4-78. Balkan Med J. 2023. PMID: 37161741 Free PMC article.
-
Filaggrin-Associated Atopic Skin, Eye, Airways, and Gut Disease, Modifying the Presentation of X-Linked Reticular Pigmentary Disorder (XLPDR).J Clin Immunol. 2024 Jan 2;44(1):38. doi: 10.1007/s10875-023-01637-x. J Clin Immunol. 2024. PMID: 38165470
-
Scleroderma specific autoantibodies in rheumatoid arthritis and Sjögren's syndrome patients with interstitial lung disease: Prevalence and associations.J Transl Autoimmun. 2022 Dec 22;6:100183. doi: 10.1016/j.jtauto.2022.100183. eCollection 2023. J Transl Autoimmun. 2022. PMID: 36619654 Free PMC article.
-
The world of rare interstitial lung diseases.Eur Respir Rev. 2023 Feb 7;32(167):220161. doi: 10.1183/16000617.0161-2022. Print 2023 Mar 31. Eur Respir Rev. 2023. PMID: 36754433 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
