Brain-derived neurotropic factor mediates neuroprotection of mesenchymal stem cell-derived extracellular vesicles against severe intraventricular hemorrhage in newborn rats
- PMID: 33319929
- PMCID: PMC7900593
- DOI: 10.1002/sctm.20-0301
Brain-derived neurotropic factor mediates neuroprotection of mesenchymal stem cell-derived extracellular vesicles against severe intraventricular hemorrhage in newborn rats
Abstract
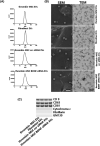
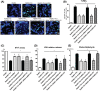
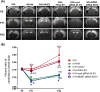
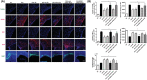
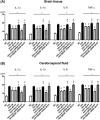
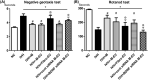
Brain-derived neurotropic factor (BDNF), which is secreted by mesenchymal stem cells (MSCs), protects against severe intraventricular hemorrhage (IVH)-induced brain injuries. Although the paracrine protective effects of MSCs are mediated primarily by extracellular vesicles (EVs), the therapeutic efficacy of MSC-derived EVs and the role of the BDNF in the EVs have not been studied. This study aimed to determine whether MSC-derived EVs attenuate severe IVH-induced brain injuries, and if so, whether this protection is mediated by BDNF transfer. We compared the therapeutic efficacy of MSCs, MSC-derived EVs with or without BDNF knockdown, and fibroblast-derived EVs in vitro in rat cortical neuronal cells challenged with thrombin and in vivo in newborn rats by injecting 200 μL of blood at postnatal day (P) 4 and transplanting 1 × 105 MSCs or 20 μg of EVs at P6. The MSCs and MSC-derived EVs, but not the EVs derived from BDNF-knockdown MSCs or fibroblasts, significantly attenuated in vitro thrombin-induced neuronal cell death and in vivo severe IVH-induced brain injuries such as increased neuronal cell death, astrogliosis, and inflammatory responses; reduced myelin basic protein and neurogenesis; led to progression of posthemorrhagic hydrocephalus; and impaired behavioral test performance. Our data indicate that MSC-derived EVs are as effective as parental MSCs in attenuating severe IVH-induced brain injuries, and this neuroprotection is primarily mediated by BDNF transfer via EVs.
Keywords: brain-derived neurotropic factor; extracellular vesicles; intraventricular hemorrhage; mesenchymal stem cells.
© 2020 The Authors. STEM CELLS TRANSLATIONAL MEDICINE published by Wiley Periodicals LLC on behalf of AlphaMed Press.
Conflict of interest statement
W.S.P., Y.S.C., D.K.S., and S.Y.A. declared potential conflicts of interest arising from a filed or issued patent titled “Pharmaceutical composition for treating cerebrovascular diseases, containing stem cell‐derived exosome as active ingredient” as coinventors, not as patentees. The other authors declared no potential conflicts of interest.
Figures
Similar articles
-
Pivotal Role of Brain-Derived Neurotrophic Factor Secreted by Mesenchymal Stem Cells in Severe Intraventricular Hemorrhage in Newborn Rats.Cell Transplant. 2017 Jan 24;26(1):145-156. doi: 10.3727/096368916X692861. Epub 2016 Aug 16. Cell Transplant. 2017. PMID: 27535166 Free PMC article.
-
Human UCB-MSCs treatment upon intraventricular hemorrhage contributes to attenuate hippocampal neuron loss and circuit damage through BDNF-CREB signaling.Stem Cell Res Ther. 2018 Nov 21;9(1):326. doi: 10.1186/s13287-018-1052-5. Stem Cell Res Ther. 2018. PMID: 30463591 Free PMC article.
-
Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury.Exp Mol Med. 2018 Apr 13;50(4):1-12. doi: 10.1038/s12276-018-0055-8. Exp Mol Med. 2018. PMID: 29650962 Free PMC article.
-
Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles as novel cell-free therapy for treatment of autoimmune disorders.Exp Mol Pathol. 2021 Feb;118:104566. doi: 10.1016/j.yexmp.2020.104566. Epub 2020 Nov 6. Exp Mol Pathol. 2021. PMID: 33160961 Review.
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles in Tissue Regeneration.Cell Transplant. 2020 Jan-Dec;29:963689720908500. doi: 10.1177/0963689720908500. Cell Transplant. 2020. PMID: 32207341 Free PMC article. Review.
Cited by
-
Anti-inflammatory effects of antenatal administration of stem cell derived extracellular vesicles in the brain of rat fetuses with congenital diaphragmatic hernia.Pediatr Surg Int. 2023 Nov 13;39(1):291. doi: 10.1007/s00383-023-05578-9. Pediatr Surg Int. 2023. PMID: 37955723
-
Emerging role of mesenchymal stromal cells (MSCs)-derived exosome in neurodegeneration-associated conditions: a groundbreaking cell-free approach.Stem Cell Res Ther. 2022 Aug 19;13(1):423. doi: 10.1186/s13287-022-03122-5. Stem Cell Res Ther. 2022. PMID: 35986375 Free PMC article. Review.
-
Proficiency of Extracellular Vesicles From hiPSC-Derived Neural Stem Cells in Modulating Proinflammatory Human Microglia: Role of Pentraxin-3 and miRNA-21-5p.Front Mol Neurosci. 2022 May 17;15:845542. doi: 10.3389/fnmol.2022.845542. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35656007 Free PMC article.
-
Progress in Research on Stem Cells in Neonatal Refractory Diseases.J Pers Med. 2023 Aug 21;13(8):1281. doi: 10.3390/jpm13081281. J Pers Med. 2023. PMID: 37623531 Free PMC article. Review.
-
A Review of the Use of Extracellular Vesicles in the Treatment of Neonatal Diseases: Current State and Problems with Translation to the Clinic.Int J Mol Sci. 2024 Mar 1;25(5):2879. doi: 10.3390/ijms25052879. Int J Mol Sci. 2024. PMID: 38474125 Free PMC article. Review.
References
-
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529‐534. - PubMed
-
- Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993‐1994. Pediatrics. 2000;105:1216‐1226. - PubMed
-
- International randomised controlled trial of acetazolamide and furosemide in posthaemorrhagic ventricular dilatation in infancy. International PHVD Drug Trial Group. Lancet. 1998;352:433‐440. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

