Therapeutic genetic variation revealed in diverse Hsp104 homologs
- PMID: 33319748
- PMCID: PMC7785292
- DOI: 10.7554/eLife.57457
Therapeutic genetic variation revealed in diverse Hsp104 homologs
Abstract
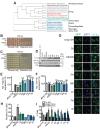
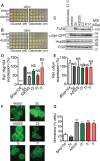
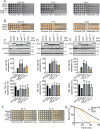
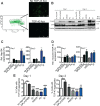
The AAA+ protein disaggregase, Hsp104, increases fitness under stress by reversing stress-induced protein aggregation. Natural Hsp104 variants might exist with enhanced, selective activity against neurodegenerative disease substrates. However, natural Hsp104 variation remains largely unexplored. Here, we screened a cross-kingdom collection of Hsp104 homologs in yeast proteotoxicity models. Prokaryotic ClpG reduced TDP-43, FUS, and α-synuclein toxicity, whereas prokaryotic ClpB and hyperactive variants were ineffective. We uncovered therapeutic genetic variation among eukaryotic Hsp104 homologs that specifically antagonized TDP-43 condensation and toxicity in yeast and TDP-43 aggregation in human cells. We also uncovered distinct eukaryotic Hsp104 homologs that selectively antagonized α-synuclein condensation and toxicity in yeast and dopaminergic neurodegeneration in C. elegans. Surprisingly, this therapeutic variation did not manifest as enhanced disaggregase activity, but rather as increased passive inhibition of aggregation of specific substrates. By exploring natural tuning of this passive Hsp104 activity, we elucidated enhanced, substrate-specific agents that counter proteotoxicity underlying neurodegeneration.
Keywords: C. elegans; Hsp104; S. cerevisiae; TDP-43; alpha-synuclein; biochemistry; chaperone; chemical biology; disaggregase; genetics; genomics; protein misfolding.
© 2020, March et al.
Conflict of interest statement
ZM, KS, HK, XY, LC, MJ, JL, EC, EG, CW, KM, RJ, AJ, KC, GC, OS No competing interests declared, JS JS is a consultant for Dewpoint Therapeutics and Maze Therapeutics.
Figures















Similar articles
-
Potentiated Hsp104 variants antagonize diverse proteotoxic misfolding events.Cell. 2014 Jan 16;156(1-2):170-82. doi: 10.1016/j.cell.2013.11.047. Cell. 2014. PMID: 24439375 Free PMC article.
-
Mining Disaggregase Sequence Space to Safely Counter TDP-43, FUS, and α-Synuclein Proteotoxicity.Cell Rep. 2019 Aug 20;28(8):2080-2095.e6. doi: 10.1016/j.celrep.2019.07.069. Cell Rep. 2019. PMID: 31433984 Free PMC article.
-
Tuning Hsp104 specificity to selectively detoxify α-synuclein.Mol Cell. 2023 Sep 21;83(18):3314-3332.e9. doi: 10.1016/j.molcel.2023.07.029. Epub 2023 Aug 24. Mol Cell. 2023. PMID: 37625404 Free PMC article.
-
Engineering therapeutic protein disaggregases.Mol Biol Cell. 2016 May 15;27(10):1556-60. doi: 10.1091/mbc.E15-10-0693. Mol Biol Cell. 2016. PMID: 27255695 Free PMC article. Review.
-
Engineering enhanced protein disaggregases for neurodegenerative disease.Prion. 2015;9(2):90-109. doi: 10.1080/19336896.2015.1020277. Prion. 2015. PMID: 25738979 Free PMC article. Review.
Cited by
-
Cytoplasmic molecular chaperones in Pseudomonas species.J Microbiol. 2022 Nov;60(11):1049-1060. doi: 10.1007/s12275-022-2425-0. Epub 2022 Nov 1. J Microbiol. 2022. PMID: 36318358 Review.
-
(Dis)Solving the problem of aberrant protein states.Dis Model Mech. 2021 May 1;14(5):dmm048983. doi: 10.1242/dmm.048983. Epub 2021 May 4. Dis Model Mech. 2021. PMID: 33942880 Free PMC article. Review.
-
Regulating Phase Transition in Neurodegenerative Diseases by Nuclear Import Receptors.Biology (Basel). 2022 Jul 4;11(7):1009. doi: 10.3390/biology11071009. Biology (Basel). 2022. PMID: 36101390 Free PMC article. Review.
-
Overexpression of Hsp104 by Causing Dissolution of the Prion Seeds Cures the Yeast [PSI+] Prion.Int J Mol Sci. 2023 Jun 29;24(13):10833. doi: 10.3390/ijms241310833. Int J Mol Sci. 2023. PMID: 37446010 Free PMC article.
-
Hsp104 N-terminal domain interaction with substrates plays a regulatory role in protein disaggregation.FEBS J. 2022 Sep;289(17):5359-5377. doi: 10.1111/febs.16441. Epub 2022 Mar 30. FEBS J. 2022. PMID: 35305079 Free PMC article.
References
-
- Armakola M, Higgins MJ, Figley MD, Barmada SJ, Scarborough EA, Diaz Z, Fang X, Shorter J, Krogan NJ, Finkbeiner S, Farese RV, Gitler AD. Inhibition of RNA lariat debranching enzyme suppresses TDP-43 toxicity in ALS disease models. Nature Genetics. 2012;44:1302–1309. doi: 10.1038/ng.2434. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

