Pre-existing influenza-specific nasal IgA or nasal viral infection does not affect live attenuated influenza vaccine immunogenicity in children
- PMID: 33314126
- PMCID: PMC7944357
- DOI: 10.1111/cei.13564
Pre-existing influenza-specific nasal IgA or nasal viral infection does not affect live attenuated influenza vaccine immunogenicity in children
Abstract
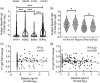
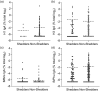
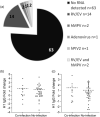
The United Kingdom has a national immunization programme which includes annual influenza vaccination in school-aged children, using live attenuated influenza vaccine (LAIV). LAIV is given annually, and it is unclear whether repeat administration can affect immunogenicity. Because LAIV is delivered intranasally, pre-existing local antibody might be important. In this study, we analysed banked samples from a study performed during the 2017/18 influenza season to investigate the role of pre-existing influenza-specific nasal immunoglobulin (Ig)A in children aged 6-14 years. Nasopharyngeal swabs were collected prior to LAIV immunization to measure pre-existing IgA levels and test for concurrent upper respiratory tract viral infections (URTI). Oral fluid samples were taken at baseline and 21-28 days after LAIV to measure IgG as a surrogate of immunogenicity. Antibody levels at baseline were compared with a pre-existing data set of LAIV shedding from the same individuals, measured by reverse transcription-polymerase chain reaction. There was detectable nasal IgA specific to all four strains in the vaccine at baseline. However, baseline nasal IgA did not correlate with the fold change in IgG response to the vaccine. Baseline nasal IgA also did not have an impact upon whether vaccine virus RNA was detectable after immunization. There was no difference in fold change of antibody between individuals with and without an URTI at the time of immunization. Overall, we observed no effect of pre-existing influenza-specific nasal antibody levels on immunogenicity, supporting annual immunization with LAIV in children.
Keywords: IgA; LAIV; influenza; mucosal; schoolchildren.
© 2021 British Society for Immunology.
Conflict of interest statement
The authors have no competing interests.
Figures
Similar articles
-
Immunogenicity and Viral Shedding of Russian-Backbone, Seasonal, Trivalent, Live, Attenuated Influenza Vaccine in a Phase II, Randomized, Placebo-Controlled Trial Among Preschool-Aged Children in Urban Bangladesh.Clin Infect Dis. 2019 Aug 16;69(5):777-785. doi: 10.1093/cid/ciy1003. Clin Infect Dis. 2019. PMID: 30481272 Free PMC article. Clinical Trial.
-
Differences in nasal immunoglobulin A responses to influenza vaccine strains after live attenuated influenza vaccine (LAIV) immunization in children.Clin Exp Immunol. 2020 Feb;199(2):109-118. doi: 10.1111/cei.13395. Epub 2019 Nov 15. Clin Exp Immunol. 2020. PMID: 31670841 Free PMC article. Clinical Trial.
-
Localized mucosal response to intranasal live attenuated influenza vaccine in adults.J Infect Dis. 2013 Jan 1;207(1):115-24. doi: 10.1093/infdis/jis641. Epub 2012 Oct 19. J Infect Dis. 2013. PMID: 23087433 Free PMC article.
-
Live attenuated influenza vaccine (FluMist®; Fluenz™): a review of its use in the prevention of seasonal influenza in children and adults.Drugs. 2011 Aug 20;71(12):1591-622. doi: 10.2165/11206860-000000000-00000. Drugs. 2011. PMID: 21861544 Review.
-
Intranasal Inactivated Influenza Vaccines: a Reasonable Approach to Improve the Efficacy of Influenza Vaccine?Jpn J Infect Dis. 2016;69(3):165-79. doi: 10.7883/yoken.JJID.2015.560. Jpn J Infect Dis. 2016. PMID: 27212584 Review.
Cited by
-
Influenza vaccine format mediates distinct cellular and antibody responses in human immune organoids.Immunity. 2023 Aug 8;56(8):1910-1926.e7. doi: 10.1016/j.immuni.2023.06.019. Epub 2023 Jul 20. Immunity. 2023. PMID: 37478854 Free PMC article.
-
Inno4Vac Workshop Report Part 1: Controlled Human Influenza Virus Infection Model (CHIVIM) Strain Selection and Immune Assays for CHIVIM Studies, November 2021, MHRA, UK.Influenza Other Respir Viruses. 2024 Nov;18(11):e70014. doi: 10.1111/irv.70014. Influenza Other Respir Viruses. 2024. PMID: 39496425 Free PMC article. Review.
-
Factors associated with viral RNA shedding and evaluation of potential viral infectivity at returning to school in influenza outpatients after treatment with baloxavir marboxil and neuraminidase inhibitors during 2013/2014-2019/2020 seasons in Japan: an observational study.BMC Infect Dis. 2023 Mar 29;23(1):188. doi: 10.1186/s12879-023-08140-z. BMC Infect Dis. 2023. PMID: 36991360 Free PMC article.
-
Mucosal vaccines - fortifying the frontiers.Nat Rev Immunol. 2022 Apr;22(4):236-250. doi: 10.1038/s41577-021-00583-2. Epub 2021 Jul 26. Nat Rev Immunol. 2022. PMID: 34312520 Free PMC article. Review.
-
Early mucosal events promote distinct mucosal and systemic antibody responses to live attenuated influenza vaccine.Nat Commun. 2023 Dec 5;14(1):8053. doi: 10.1038/s41467-023-43842-7. Nat Commun. 2023. PMID: 38052824 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

