Galectin-9 expression defines exhausted T cells and impaired cytotoxic NK cells in patients with virus-associated solid tumors
- PMID: 33310773
- PMCID: PMC7735134
- DOI: 10.1136/jitc-2020-001849
Galectin-9 expression defines exhausted T cells and impaired cytotoxic NK cells in patients with virus-associated solid tumors
Abstract
Background: We have previously reported that the upregulation of galectin-9 (Gal-9) on CD4+ and CD8+ T cells in HIV patients was associated with impaired T cell effector functions. Gal-9 is a ligand for T cell immunoglobulin and mucin domain-3, and its expression on T cells in cancer has not been investigated. Therefore, we aimed to investigate the expression level and effects of Gal-9 on T cell functions in patients with virus-associated solid tumors (VASTs).
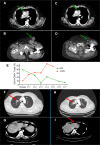
Methods: 40 patients with VASTs through a non-randomized and biomarker-driven phase II LATENT trial were investigated. Peripheral blood mononuclear cells and tumor biopsies were obtained and subjected to immunophenotyping. In this trial, the effects of oral valproate and avelumab (anti-PD-L1) was investigated in regards to the expression of Gal-9 on T cells.
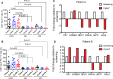
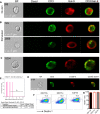
Results: We report the upregulation of Gal-9 expression by peripheral and tumor-infiltrating CD4+ and CD8+ T lymphocytes in patients with VASTs. Our results indicate that Gal-9 expression is associated with dysfunctional T cell effector functions in the periphery and tumor microenvironment (TME). Coexpression of Gal-9 with PD-1 or T cell immunoglobulin and ITIM domain (TIGIT) exhibited a synergistic inhibitory effect and enhanced an exhausted T cell phenotype. Besides, responding patients to treatment had lower Gal-9 mRNA expression in the TME. Translocation of Gal-9 from the cytosol to the cell membrane of T cells following stimulation suggests persistent T cell receptor (TCR) stimulation as a potential contributing factor in Gal-9 upregulation in patients with VASTs. Moreover, partial colocalization of Gal-9 with CD3 on T cells likely impacts the initiation of signal transduction via TCR as shown by the upregulation of ZAP70 in Gal-9+ T cells. Also, we found an expansion of Gal-9+ but not TIGIT+ NK cells in patients with VASTs; however, dichotomous to TIGIT+ NK cells, Gal-9+ NK cells exhibited impaired cytotoxic molecules but higher Interferon gamma (IFN-γ) expression.
Conclusion: Our data indicate that higher Gal-9-expressing CD8+ T cells were associated with poor prognosis following immunotherapy with anti-Programmed death-ligand 1 (PD-L1) (avelumab) in our patients' cohort. Therefore, for the very first time to our knowledge, we report Gal-9 as a novel marker of T cell exhaustion and the potential target of immunotherapy in patients with VASTs.
Keywords: T-lymphocytes; immunologic; investigational; killer cells; natural; receptors; therapies; tumor microenvironment.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




Similar articles
-
Galectin-9 and VISTA Expression Define Terminally Exhausted T Cells in HIV-1 Infection.J Immunol. 2020 May 1;204(9):2474-2491. doi: 10.4049/jimmunol.1901481. Epub 2020 Mar 23. J Immunol. 2020. PMID: 32205423
-
Galectin-9 Expression Defines a Subpopulation of NK Cells with Impaired Cytotoxic Effector Molecules but Enhanced IFN-γ Production, Dichotomous to TIGIT, in HIV-1 Infection.Immunohorizons. 2019 Nov 15;3(11):531-546. doi: 10.4049/immunohorizons.1900087. Immunohorizons. 2019. PMID: 31732662
-
Immunosuppression of Human Adipose-Derived Stem Cells on T Cell Subsets via the Reduction of NF-kappaB Activation Mediated by PD-L1/PD-1 and Gal-9/TIM-3 Pathways.Stem Cells Dev. 2018 Sep 1;27(17):1191-1202. doi: 10.1089/scd.2018.0033. Stem Cells Dev. 2018. PMID: 29978730
-
CD155/TIGIT, a novel immune checkpoint in human cancers (Review).Oncol Rep. 2021 Mar;45(3):835-845. doi: 10.3892/or.2021.7943. Epub 2021 Jan 19. Oncol Rep. 2021. PMID: 33469677 Review.
-
CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review.J Cell Physiol. 2019 Jun;234(6):8509-8521. doi: 10.1002/jcp.27782. Epub 2018 Nov 22. J Cell Physiol. 2019. PMID: 30520029 Review.
Cited by
-
Galectin-9, a Player in Cytokine Release Syndrome and a Surrogate Diagnostic Biomarker in SARS-CoV-2 Infection.mBio. 2021 May 4;12(3):e00384-21. doi: 10.1128/mBio.00384-21. mBio. 2021. PMID: 33947753 Free PMC article.
-
Galectin-9 blockade synergizes with ATM inhibition to induce potent anti-tumor immunity.Int J Biol Sci. 2023 Jan 16;19(3):981-993. doi: 10.7150/ijbs.79852. eCollection 2023. Int J Biol Sci. 2023. PMID: 36778120 Free PMC article.
-
Analysis of SARS-CoV-2 isolates, namely the Wuhan strain, Delta variant, and Omicron variant, identifies differential immune profiles.Microbiol Spectr. 2023 Sep 7;11(5):e0125623. doi: 10.1128/spectrum.01256-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 37676005 Free PMC article.
-
Dynamic immune recovery process after liver transplantation revealed by single-cell multi-omics analysis.Innovation (Camb). 2024 Mar 1;5(3):100599. doi: 10.1016/j.xinn.2024.100599. eCollection 2024 May 6. Innovation (Camb). 2024. PMID: 38510071 Free PMC article.
-
Killer instincts: natural killer cells as multifactorial cancer immunotherapy.Front Immunol. 2023 Nov 28;14:1269614. doi: 10.3389/fimmu.2023.1269614. eCollection 2023. Front Immunol. 2023. PMID: 38090565 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
