Disturbed Presynaptic Ca2+ Signaling in Photoreceptors in the EAE Mouse Model of Multiple Sclerosis
- PMID: 33305185
- PMCID: PMC7711289
- DOI: 10.1016/j.isci.2020.101830
Disturbed Presynaptic Ca2+ Signaling in Photoreceptors in the EAE Mouse Model of Multiple Sclerosis
Abstract
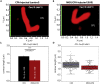
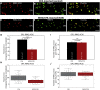
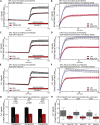
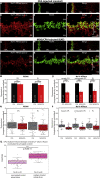
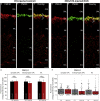
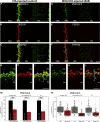
Multiple sclerosis (MS) is a demyelinating disease caused by an auto-reactive immune system. Recent studies also demonstrated synapse dysfunctions in MS patients and MS mouse models. We previously observed decreased synaptic vesicle exocytosis in photoreceptor synapses in the EAE mouse model of MS at an early, preclinical stage. In the present study, we analyzed whether synaptic defects are associated with altered presynaptic Ca2+ signaling. Using high-resolution immunolabeling, we found a reduced signal intensity of Cav-channels and RIM2 at active zones in early, preclinical EAE. In line with these morphological alterations, depolarization-evoked increases of presynaptic Ca2+ were significantly smaller. In contrast, basal presynaptic Ca2+ was elevated. We observed a decreased expression of Na+/K+-ATPase and plasma membrane Ca2+ ATPase 2 (PMCA2), but not PMCA1, in photoreceptor terminals of EAE mice that could contribute to elevated basal Ca2+. Thus, complex Ca2+ signaling alterations contribute to synaptic dysfunctions in photoreceptors in early EAE.
Keywords: Biological Sciences; Cell Biology; Cellular Neuroscience; Molecular Biology; Molecular Neuroscience; Neuroscience.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Early Changes in Exo- and Endocytosis in the EAE Mouse Model of Multiple Sclerosis Correlate with Decreased Synaptic Ribbon Size and Reduced Ribbon-Associated Vesicle Pools in Rod Photoreceptor Synapses.Int J Mol Sci. 2021 Oct 6;22(19):10789. doi: 10.3390/ijms221910789. Int J Mol Sci. 2021. PMID: 34639129 Free PMC article.
-
Analysis of RIM Expression and Function at Mouse Photoreceptor Ribbon Synapses.J Neurosci. 2017 Aug 16;37(33):7848-7863. doi: 10.1523/JNEUROSCI.2795-16.2017. Epub 2017 Jul 12. J Neurosci. 2017. PMID: 28701482 Free PMC article.
-
RIM1/2-Mediated Facilitation of Cav1.4 Channel Opening Is Required for Ca2+-Stimulated Release in Mouse Rod Photoreceptors.J Neurosci. 2015 Sep 23;35(38):13133-47. doi: 10.1523/JNEUROSCI.0658-15.2015. J Neurosci. 2015. PMID: 26400943 Free PMC article.
-
Presynaptic excitability.Int Rev Neurobiol. 1995;38:201-51. doi: 10.1016/s0074-7742(08)60527-9. Int Rev Neurobiol. 1995. PMID: 8537201 Review.
-
Presynaptic calcium channels.Neurosci Res. 2018 Feb;127:33-44. doi: 10.1016/j.neures.2017.09.012. Epub 2018 Jan 6. Neurosci Res. 2018. PMID: 29317246 Review.
Cited by
-
RIBEYE B-Domain Is Essential for RIBEYE A-Domain Stability and Assembly of Synaptic Ribbons.Front Mol Neurosci. 2022 Jan 28;15:838311. doi: 10.3389/fnmol.2022.838311. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35153673 Free PMC article.
-
Neuropathic Pain in Multiple Sclerosis and Its Animal Models: Focus on Mechanisms, Knowledge Gaps and Future Directions.Front Neurol. 2021 Dec 16;12:793745. doi: 10.3389/fneur.2021.793745. eCollection 2021. Front Neurol. 2021. PMID: 34975739 Free PMC article. Review.
-
Na+/K+-ATPase: ion pump, signal transducer, or cytoprotective protein, and novel biological functions.Neural Regen Res. 2024 Dec 1;19(12):2684-2697. doi: 10.4103/NRR.NRR-D-23-01175. Epub 2024 Jan 31. Neural Regen Res. 2024. PMID: 38595287 Free PMC article.
-
Rabconnectin-3α/DMXL2 Is Locally Enriched at the Synaptic Ribbon of Rod Photoreceptor Synapses.Cells. 2023 Jun 19;12(12):1665. doi: 10.3390/cells12121665. Cells. 2023. PMID: 37371135 Free PMC article.
-
Early Changes in Exo- and Endocytosis in the EAE Mouse Model of Multiple Sclerosis Correlate with Decreased Synaptic Ribbon Size and Reduced Ribbon-Associated Vesicle Pools in Rod Photoreceptor Synapses.Int J Mol Sci. 2021 Oct 6;22(19):10789. doi: 10.3390/ijms221910789. Int J Mol Sci. 2021. PMID: 34639129 Free PMC article.
References
-
- Amaral E., Leite L.F., Gomez M.V., Prado M.A.M., Guatimosim C. Ouabain evokes exocytosis dependent on ryanodine and mitochondrial calcium stores that is not followed by compensatory endocytosis at the neuromuscular junction. Neurochem. Int. 2009;55:406–413. - PubMed
-
- Aperia A. New roles for an old enzyme: Na,K-ATPase emerges as an interesting drug target. J. Intern. Med. 2007;261:44–52. - PubMed
-
- Ball S.L., Powers P.A., Shin H.S., Morgans C.W., Peachey N.S., Gregg R.G. Role of the beta2 subunit of voltage-gated channels in the retinal outer plexiform layer. Invest. Ophthalmol. Vis. Sci. 2002;43:1595–1603. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

