Ferroptosis, a Regulated Neuronal Cell Death Type After Intracerebral Hemorrhage
- PMID: 33304242
- PMCID: PMC7701249
- DOI: 10.3389/fncel.2020.591874
Ferroptosis, a Regulated Neuronal Cell Death Type After Intracerebral Hemorrhage
Abstract
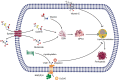
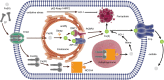
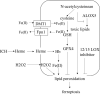
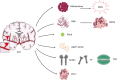
Ferroptosis is a term that describes one form of regulated non-apoptotic cell death. It is triggered by the iron-dependent accumulation of lipid peroxides. Emerging evidence suggests a link between ferroptosis and the pathophysiological processes of neurological disorders, including stroke, degenerative diseases, neurotrauma, and cancer. Hemorrhagic stroke, also known as intracerebral hemorrhage (ICH), belongs to a devastating illness for its high level in morbidity and mortality. Currently, there are few established treatments and limited knowledge about the mechanisms of post-ICH neuronal death. The secondary brain damage after ICH is mainly attributed to oxidative stress and hemoglobin lysate, including iron, which leads to irreversible damage to neurons. Therefore, ferroptosis is becoming a common trend in research of neuronal death after ICH. Accumulative data suggest that the inhibition of ferroptosis may effectively prevent neuronal ferroptosis, thereby reducing secondary brain damage after ICH in animal models. Ferroptosis has a close relationship with oxidative damage and iron metabolism. This review reveals the pathological pathways and regulation mechanism of ferroptosis following ICH and then offers potential intervention strategies to mitigate neuron death and dysfunction after ICH.
Keywords: antioxidation; ferroptosis; intracerebral hemorrhage; iron metabolism; lipid peroxidation.
Copyright © 2020 Bai, Liu and Wang.
Figures
Similar articles
-
Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage.Stroke Vasc Neurol. 2019 Jan 13;4(2):93-95. doi: 10.1136/svn-2018-000205. eCollection 2019 Jul. Stroke Vasc Neurol. 2019. PMID: 31338218 Free PMC article. Review.
-
Dexmedetomidine prevents hemorrhagic brain injury by reducing damage induced by ferroptosis in mice.Neurosci Lett. 2022 Sep 25;788:136842. doi: 10.1016/j.neulet.2022.136842. Epub 2022 Aug 19. Neurosci Lett. 2022. PMID: 35995304
-
Neuronal ferroptosis after intracerebral hemorrhage.Front Mol Biosci. 2022 Aug 5;9:966478. doi: 10.3389/fmolb.2022.966478. eCollection 2022. Front Mol Biosci. 2022. PMID: 35992267 Free PMC article. Review.
-
Nox4-and Tf/TfR-mediated peroxidation and iron overload exacerbate neuronal ferroptosis after intracerebral hemorrhage: Involvement of EAAT3 dysfunction.Free Radic Biol Med. 2023 Apr;199:67-80. doi: 10.1016/j.freeradbiomed.2023.02.015. Epub 2023 Feb 18. Free Radic Biol Med. 2023. PMID: 36805044
-
Mitochondrial Inhibitor Rotenone Triggers and Enhances Neuronal Ferroptosis Following Intracerebral Hemorrhage.ACS Chem Neurosci. 2023 Mar 15;14(6):1071-1079. doi: 10.1021/acschemneuro.2c00308. Epub 2023 Feb 27. ACS Chem Neurosci. 2023. PMID: 36848438
Cited by
-
Pivotal Role of GSTO2 in Ferroptotic Neuronal Injury After Intracerebral Hemorrhage.J Mol Neurosci. 2024 Feb 22;74(1):24. doi: 10.1007/s12031-023-02187-y. J Mol Neurosci. 2024. PMID: 38386166 Free PMC article.
-
Withaferin A inhibits ferroptosis and protects against intracerebral hemorrhage.Neural Regen Res. 2023 Jun;18(6):1308-1315. doi: 10.4103/1673-5374.355822. Neural Regen Res. 2023. PMID: 36453416 Free PMC article.
-
Methyltransferase-like 3 silenced inhibited the ferroptosis development via regulating the glutathione peroxidase 4 levels in the intracerebral hemorrhage progression.Bioengineered. 2022 Jun;13(6):14215-14226. doi: 10.1080/21655979.2022.2084494. Bioengineered. 2022. PMID: 35758287 Free PMC article.
-
Lipid peroxidation-induced ferroptosis as a therapeutic target for mitigating neuronal injury and inflammation in sepsis-associated encephalopathy: insights into the hippocampal PEBP-1/15-LOX/GPX4 pathway.Lipids Health Dis. 2024 Apr 29;23(1):128. doi: 10.1186/s12944-024-02116-x. Lipids Health Dis. 2024. PMID: 38685023 Free PMC article.
-
20-HETE Participates in Intracerebral Hemorrhage-Induced Acute Injury by Promoting Cell Ferroptosis.Front Neurol. 2021 Nov 12;12:763419. doi: 10.3389/fneur.2021.763419. eCollection 2021. Front Neurol. 2021. PMID: 34867747 Free PMC article.
References
-
- Bamford J., Sandercock P., Dennis M., Burn J., Warlow C. (1990). A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project—1981–86. 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry 53, 16–22. 10.1136/jnnp.53.1.16 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

