TFEB/Mitf links impaired nuclear import to autophagolysosomal dysfunction in C9-ALS
- PMID: 33300868
- PMCID: PMC7758070
- DOI: 10.7554/eLife.59419
TFEB/Mitf links impaired nuclear import to autophagolysosomal dysfunction in C9-ALS
Abstract
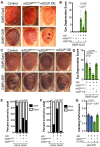
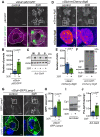
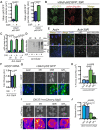
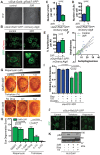
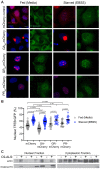
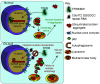
Disrupted nucleocytoplasmic transport (NCT) has been implicated in neurodegenerative disease pathogenesis; however, the mechanisms by which disrupted NCT causes neurodegeneration remain unclear. In a Drosophila screen, we identified ref(2)P/p62, a key regulator of autophagy, as a potent suppressor of neurodegeneration caused by the GGGGCC hexanucleotide repeat expansion (G4C2 HRE) in C9orf72 that causes amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). We found that p62 is increased and forms ubiquitinated aggregates due to decreased autophagic cargo degradation. Immunofluorescence and electron microscopy of Drosophila tissues demonstrate an accumulation of lysosome-like organelles that precedes neurodegeneration. These phenotypes are partially caused by cytoplasmic mislocalization of Mitf/TFEB, a key transcriptional regulator of autophagolysosomal function. Additionally, TFEB is mislocalized and downregulated in human cells expressing GGGGCC repeats and in C9-ALS patient motor cortex. Our data suggest that the C9orf72-HRE impairs Mitf/TFEB nuclear import, thereby disrupting autophagy and exacerbating proteostasis defects in C9-ALS/FTD.
Keywords: D. melanogaster; amyotrophic lateral sclerosis; autophagy; c9orf72; cell biology; lysosome; neuroscience; nuclear pore; nucleocytoplasmic transport.
© 2020, Cunningham et al.
Conflict of interest statement
KC, KM, KR, MS, JG, HS, ZZ, HS, JG, SD, JR, KZ, TL No competing interests declared, HB Reviewing editor, eLife
Figures



Similar articles
-
C9orf72 ALS-FTD: recent evidence for dysregulation of the autophagy-lysosome pathway at multiple levels.Autophagy. 2021 Nov;17(11):3306-3322. doi: 10.1080/15548627.2021.1872189. Epub 2021 Feb 26. Autophagy. 2021. PMID: 33632058 Free PMC article. Review.
-
The C9orf72 repeat expansion disrupts nucleocytoplasmic transport.Nature. 2015 Sep 3;525(7567):56-61. doi: 10.1038/nature14973. Epub 2015 Aug 26. Nature. 2015. PMID: 26308891 Free PMC article.
-
Systemic deregulation of autophagy upon loss of ALS- and FTD-linked C9orf72.Autophagy. 2017 Jul 3;13(7):1254-1255. doi: 10.1080/15548627.2017.1299312. Autophagy. 2017. PMID: 28319438 Free PMC article.
-
Disrupted endoplasmic reticulum-mediated autophagosomal biogenesis in a Drosophila model of C9-ALS-FTD.Autophagy. 2024 Jan;20(1):94-113. doi: 10.1080/15548627.2023.2249750. Epub 2023 Aug 27. Autophagy. 2024. PMID: 37599467 Free PMC article.
-
Nucleocytoplasmic transport in C9orf72-mediated ALS/FTD.Nucleus. 2016 Apr 25;7(2):132-7. doi: 10.1080/19491034.2016.1172152. Epub 2016 Apr 26. Nucleus. 2016. PMID: 27116041 Free PMC article. Review.
Cited by
-
Mitochondria, a Key Target in Amyotrophic Lateral Sclerosis Pathogenesis.Genes (Basel). 2023 Oct 24;14(11):1981. doi: 10.3390/genes14111981. Genes (Basel). 2023. PMID: 38002924 Free PMC article. Review.
-
A comparison study of pathological features and drug efficacy between Drosophila models of C9orf72 ALS/FTD.Mol Cells. 2024 Jan;47(1):100005. doi: 10.1016/j.mocell.2023.12.003. Epub 2023 Dec 15. Mol Cells. 2024. PMID: 38376483 Free PMC article.
-
Fly for ALS: Drosophila modeling on the route to amyotrophic lateral sclerosis modifiers.Cell Mol Life Sci. 2021 Sep;78(17-18):6143-6160. doi: 10.1007/s00018-021-03905-8. Epub 2021 Jul 28. Cell Mol Life Sci. 2021. PMID: 34322715 Free PMC article. Review.
-
Decoding Nucleotide Repeat Expansion Diseases: Novel Insights from Drosophila melanogaster Studies.Int J Mol Sci. 2024 Nov 2;25(21):11794. doi: 10.3390/ijms252111794. Int J Mol Sci. 2024. PMID: 39519345 Free PMC article. Review.
-
The prion-like effect and prion-like protein targeting strategy in amyotrophic lateral sclerosis.Heliyon. 2024 Jul 22;10(15):e34963. doi: 10.1016/j.heliyon.2024.e34963. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170125 Free PMC article. Review.
References
-
- Al-Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I, Rogelj B, Al-Chalabi A, Hortobágyi T, Shaw CE. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and Hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathologica. 2011;122:691–702. doi: 10.1007/s00401-011-0911-2. - DOI - PubMed
-
- Almeida S, Gascon E, Tran H, Chou HJ, Gendron TF, Degroot S, Tapper AR, Sellier C, Charlet-Berguerand N, Karydas A, Seeley WW, Boxer AL, Petrucelli L, Miller BL, Gao FB. Modeling key pathological features of frontotemporal dementia with C9ORF72 repeat expansion in iPSC-derived human neurons. Acta Neuropathologica. 2013;126:385–399. doi: 10.1007/s00401-013-1149-y. - DOI - PMC - PubMed
-
- Boeynaems S, Bogaert E, Michiels E, Gijselinck I, Sieben A, Jovičić A, De Baets G, Scheveneels W, Steyaert J, Cuijt I, Verstrepen KJ, Callaerts P, Rousseau F, Schymkowitz J, Cruts M, Van Broeckhoven C, Van Damme P, Gitler AD, Robberecht W, Van Den Bosch L. Drosophila screen connects nuclear transport genes to DPR pathology in c9ALS/FTD. Scientific Reports. 2016;6:20877. doi: 10.1038/srep20877. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

