Transcriptional Regulation of Cancer Immune Checkpoints: Emerging Strategies for Immunotherapy
- PMID: 33291616
- PMCID: PMC7761936
- DOI: 10.3390/vaccines8040735
Transcriptional Regulation of Cancer Immune Checkpoints: Emerging Strategies for Immunotherapy
Abstract
The study of immune evasion has gained a well-deserved eminence in cancer research by successfully developing a new class of therapeutics, immune checkpoint inhibitors, such as pembrolizumab and nivolumab, anti-PD-1 antibodies. By aiming at the immune checkpoint blockade (ICB), these new therapeutics have advanced cancer treatment with notable increases in overall survival and tumor remission. However, recent reports reveal that 40-60% of patients fail to benefit from ICB therapy due to acquired resistance or tumor relapse. This resistance may stem from increased expression of co-inhibitory immune checkpoints or alterations in the tumor microenvironment that promotes immune suppression. Because these mechanisms are poorly elucidated, the transcription factors that regulate immune checkpoints, known as "master regulators", have garnered interest. These include AP-1, IRF-1, MYC, and STAT3, which are known to regulate PD/PD-L1 and CTLA-4. Identifying these and other potential master regulators as putative therapeutic targets or biomarkers can be facilitated by mining cancer literature, public datasets, and cancer genomics resources. In this review, we describe recent advances in master regulator identification and characterization of the mechanisms underlying immune checkpoints regulation, and discuss how these master regulators of immune checkpoint molecular expression can be targeted as a form of auxiliary therapeutic strategy to complement traditional immunotherapy.
Keywords: cancer immune response; immune checkpoint inhibitor; transcription factors; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
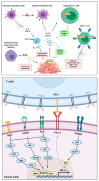
Similar articles
-
Metabolic interventions combined with CTLA-4 and PD-1/PD-L1 blockade for the treatment of tumors: mechanisms and strategies.Front Med. 2023 Oct;17(5):805-822. doi: 10.1007/s11684-023-1025-7. Epub 2023 Oct 28. Front Med. 2023. PMID: 37897562 Review.
-
Role of tumor microenvironment in the regulation of PD-L1: A novel role in resistance to cancer immunotherapy.J Cell Physiol. 2020 Oct;235(10):6496-6506. doi: 10.1002/jcp.29671. Epub 2020 Apr 2. J Cell Physiol. 2020. PMID: 32239707 Review.
-
Keeping Tumors in Check: A Mechanistic Review of Clinical Response and Resistance to Immune Checkpoint Blockade in Cancer.J Mol Biol. 2018 Jul 6;430(14):2014-2029. doi: 10.1016/j.jmb.2018.05.030. Epub 2018 May 22. J Mol Biol. 2018. PMID: 29800567 Free PMC article. Review.
-
Current Understanding of the Mechanisms Underlying Immune Evasion From PD-1/PD-L1 Immune Checkpoint Blockade in Head and Neck Cancer.Front Oncol. 2020 Feb 28;10:268. doi: 10.3389/fonc.2020.00268. eCollection 2020. Front Oncol. 2020. PMID: 32185135 Free PMC article. Review.
-
Immune checkpoints and cancer development: Therapeutic implications and future directions.Pathol Res Pract. 2021 Jul;223:153485. doi: 10.1016/j.prp.2021.153485. Epub 2021 May 15. Pathol Res Pract. 2021. PMID: 34022684 Review.
Cited by
-
The Tumor Microenvironment-Dependent Transcription Factors AHR and HIF-1α Are Dispensable for Leukemogenesis in the Eµ-TCL1 Mouse Model of Chronic Lymphocytic Leukemia.Cancers (Basel). 2021 Sep 8;13(18):4518. doi: 10.3390/cancers13184518. Cancers (Basel). 2021. PMID: 34572746 Free PMC article.
-
The generation of PD-L1 and PD-L2 in cancer cells: From nuclear chromatin reorganization to extracellular presentation.Acta Pharm Sin B. 2022 Mar;12(3):1041-1053. doi: 10.1016/j.apsb.2021.09.010. Epub 2021 Sep 16. Acta Pharm Sin B. 2022. PMID: 35530130 Free PMC article. Review.
-
TIGIT Induces (CD3+) T Cell Dysfunction in Colorectal Cancer by Inhibiting Glucose Metabolism.Front Immunol. 2021 Sep 29;12:688961. doi: 10.3389/fimmu.2021.688961. eCollection 2021. Front Immunol. 2021. PMID: 34659197 Free PMC article.
-
Network based multifactorial modelling of miRNA-target interactions.PeerJ. 2021 Mar 19;9:e11121. doi: 10.7717/peerj.11121. eCollection 2021. PeerJ. 2021. PMID: 33777541 Free PMC article.
-
Molecularly Guided Drug Repurposing for Cholangiocarcinoma: An Integrative Bioinformatic Approach.Genes (Basel). 2022 Jan 29;13(2):271. doi: 10.3390/genes13020271. Genes (Basel). 2022. PMID: 35205315 Free PMC article.
References
-
- Necchi A., Joseph R., Loriot Y., Hoffman-Censits J., Perez-Gracia J., Petrylak D., Derleth C., Tayama D., Zhu Q., Ding B., et al. Atezolizumab in platinum-treated locally advanced or metastatic urothelial carcinoma: Post-progression outcomes from the phase II IMvigor210 study. Ann. Oncol. 2017;28:3044–3050. doi: 10.1093/annonc/mdx518. - DOI - PMC - PubMed
-
- El-Khoueiry A.B., Sangro B., Yau T., Crocenzi T.S., Kudo M., Hsu C., Kim T.-Y., Choo S.-P., Trojan J., Welling T.H., et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. - DOI - PMC - PubMed
-
- Gettinger S.N., Horn L., Gandhi L., Spigel D.R., Antonia S.J., Rizvi N.A., Powderly J.D., Heist R.S., Carvajal R.D., Jackman D.M., et al. Overall Survival and Long-Term Safety of Nivolumab (Anti–Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. - DOI - PMC - PubMed
Publication types
Grants and funding
- grant number B05F630066 (R.T.)/Office of Higher Education, Science, Research and Innovation Policy Council, Thailand
- grant number B05F630082 (S.C.)/Office of Higher Education, Science, Research and Innovation Policy Council, Thailand
- grant number NDFR19/2563 (S.C.)/New Discovery and Frontier Research Grant of Mahidol University
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

