The malaria parasite sheddase SUB2 governs host red blood cell membrane sealing at invasion
- PMID: 33287958
- PMCID: PMC7723409
- DOI: 10.7554/eLife.61121
The malaria parasite sheddase SUB2 governs host red blood cell membrane sealing at invasion
Abstract
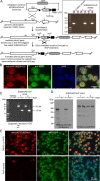
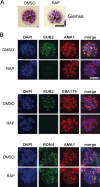
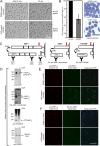
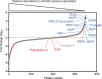
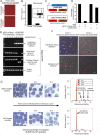
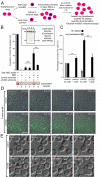
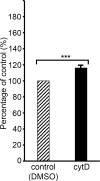
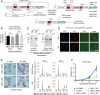
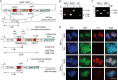
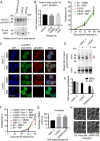
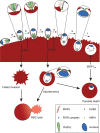
Red blood cell (RBC) invasion by malaria merozoites involves formation of a parasitophorous vacuole into which the parasite moves. The vacuole membrane seals and pinches off behind the parasite through an unknown mechanism, enclosing the parasite within the RBC. During invasion, several parasite surface proteins are shed by a membrane-bound protease called SUB2. Here we show that genetic depletion of SUB2 abolishes shedding of a range of parasite proteins, identifying previously unrecognized SUB2 substrates. Interaction of SUB2-null merozoites with RBCs leads to either abortive invasion with rapid RBC lysis, or successful entry but developmental arrest. Selective failure to shed the most abundant SUB2 substrate, MSP1, reduces intracellular replication, whilst conditional ablation of the substrate AMA1 produces host RBC lysis. We conclude that SUB2 activity is critical for host RBC membrane sealing following parasite internalisation and for correct functioning of merozoite surface proteins.
Keywords: P. falciparum; erythrocyte; infectious disease; invasion; malaria; microbiology; plasmodium; protease; sheddase.
Plain language summary
Malaria kills or disables hundreds of millions of people across the world, especially in developing economies. The most severe form of the disease is caused by Plasmodium falciparum, a single-cell parasite which, once inside a human host, forces its way into red blood cells to feed on a protein called haemoglobin. This invasion relies on P. falciparum being engulfed by the membrane of the red blood cell, which then seals off to form a compartment inside the cell where the parasite can feed and multiply. Invasion takes less than 30 seconds, and it involves P. falciparum losing the coat of proteins that covers its surface. An enzyme calls SUB2 cleaves or cuts off these proteins, but exactly why and how the shedding takes place during infection is still unclear. To investigate, Collins, Hackett et al. deactivated the gene which codes for SUB2, and examined how mutant P. falciparum would survive and multiply. Without the enzyme, the parasites failed to shed many of their proteins, including some that were not previously known to be removed by SUB2. The majority of the genetically modified parasites also failed to invade red blood cells. In particular, most of the host cells ruptured, suggesting that the protein coat needs to be discarded for the engulfing process to be completed properly. When the enzyme-free mutants did manage to make their way into a red blood cell, they starved to death because they could not digest haemoglobin. SUB2 and surface coat shedding therefore appears to be essential for the parasite to survive. P. falciparum is fast becoming resistant to the many drugs that exist to fight malaria. New treatments that target SUB2 may therefore help in combatting this deadly disease.
© 2020, Collins et al.
Conflict of interest statement
CC, FH, SH, AS, MR, LC, MB No competing interests declared
Figures



Similar articles
-
Immunization against a merozoite sheddase promotes multiple invasion of red blood cells and attenuates Plasmodium infection in mice.Malar J. 2014 Aug 12;13:313. doi: 10.1186/1475-2875-13-313. Malar J. 2014. PMID: 25115675 Free PMC article.
-
A choline-releasing glycerophosphodiesterase essential for phosphatidylcholine biosynthesis and blood stage development in the malaria parasite.Elife. 2022 Dec 28;11:e82207. doi: 10.7554/eLife.82207. Elife. 2022. PMID: 36576255 Free PMC article.
-
Juxtamembrane shedding of Plasmodium falciparum AMA1 is sequence independent and essential, and helps evade invasion-inhibitory antibodies.PLoS Pathog. 2011 Dec;7(12):e1002448. doi: 10.1371/journal.ppat.1002448. Epub 2011 Dec 15. PLoS Pathog. 2011. PMID: 22194692 Free PMC article.
-
The role of malaria merozoite proteases in red blood cell invasion.Curr Opin Microbiol. 2005 Aug;8(4):422-7. doi: 10.1016/j.mib.2005.06.018. Curr Opin Microbiol. 2005. PMID: 16019257 Review.
-
RBC membrane biomechanics and Plasmodium falciparum invasion: probing beyond ligand-receptor interactions.Trends Parasitol. 2022 Apr;38(4):302-315. doi: 10.1016/j.pt.2021.12.005. Epub 2022 Jan 4. Trends Parasitol. 2022. PMID: 34991983 Free PMC article. Review.
Cited by
-
Post-Translational Modifications of Proteins of Malaria Parasites during the Life Cycle.Int J Mol Sci. 2024 Jun 2;25(11):6145. doi: 10.3390/ijms25116145. Int J Mol Sci. 2024. PMID: 38892332 Free PMC article. Review.
-
Plasmepsin X activates the PCRCR complex of Plasmodium falciparum by processing PfRh5 for erythrocyte invasion.Nat Commun. 2023 Apr 19;14(1):2219. doi: 10.1038/s41467-023-37890-2. Nat Commun. 2023. PMID: 37072430 Free PMC article.
-
Actomyosin forces and the energetics of red blood cell invasion by the malaria parasite Plasmodium falciparum.PLoS Pathog. 2020 Oct 26;16(10):e1009007. doi: 10.1371/journal.ppat.1009007. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33104759 Free PMC article.
-
Targeting the Plasmodium falciparum proteome and organelles for potential antimalarial drug candidates.Heliyon. 2022 Aug;8(8):e10390. doi: 10.1016/j.heliyon.2022.e10390. Epub 2022 Aug 24. Heliyon. 2022. PMID: 36033316 Free PMC article. Review.
-
The AMA1-RON complex drives Plasmodium sporozoite invasion in the mosquito and mammalian hosts.PLoS Pathog. 2022 Jun 22;18(6):e1010643. doi: 10.1371/journal.ppat.1010643. eCollection 2022 Jun. PLoS Pathog. 2022. PMID: 35731833 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

