Lung Epithelial TRPA1 Mediates Lipopolysaccharide-Induced Lung Inflammation in Bronchial Epithelial Cells and Mice
- PMID: 33281629
- PMCID: PMC7705107
- DOI: 10.3389/fphys.2020.596314
Lung Epithelial TRPA1 Mediates Lipopolysaccharide-Induced Lung Inflammation in Bronchial Epithelial Cells and Mice
Abstract
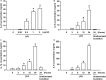
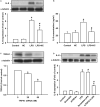
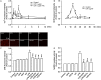
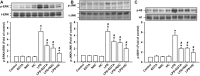
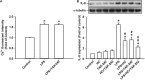
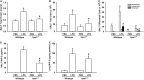
Toll-like receptor (TLR) 4 was originally thought to be the sole pattern recognition receptor for lipopolysaccharide (LPS). Transient receptor potential ankyrin 1 (TRPA1), a Ca2+-permeant channel, has been suggested as a non-TLR receptor membrane-bound sensor of LPS. We recently reported that TRPA1 is expressed in lung epithelial cells (LECs) and mediates lung inflammation induced by cigarette smoke. However, the role of TRPA1 in LPS-induced lung inflammation has not been conclusively defined, and its underlying cellular mechanisms remain unclear. In this study, our in vitro results showed that LPS sequentially produced a cascade of events, including the elevation of intracellular Ca2+, the activation of NADPH oxidase, increase in intracellular reactive oxygen species (ROS), the activation of mitogen-activated protein kinase (MAPK)/nuclear factor-kB (NF-κB) signaling, and the induction of IL-8. The increase in intracellular Ca2+ was inhibited by HC030031 (a TRPA1 antagonist) but was unaffected by TAK-242 (a TLR-4 inhibitor). The activation of NADPH oxidase was prevented by its inhibitor apocynin, EGTA (an extracellular Ca2+ chelator), and HC030031. The increase in intracellular ROS was attenuated by apocynin, N-acetyl-cysteine (NAC, a ROS scavenger), EGTA, and HC030031. The activation of the MAPK/NF-κB signaling was halted by NAC, EGTA, and HC030031. IL-8 induction was suppressed by HC030031 and TRPA1 siRNA, and further reduced by the combination of HC030031 and TAK-242. Our in vivo studies showed that trpa1-/- mice exhibited a reduced level of LPS-induced lung inflammation compared with wild-type mice as evidenced by the alleviations of increases in vascular permeability, inflammatory cell infiltration, inflammatory cytokine levels, oxidative stress, and MAPK signaling activation. Thus, in LECs, LPS may activate TRPA1 resulting in an increase in Ca2+ influx. The increased intracellular Ca2+ leads to NADPH oxidase activation, which causes an increase in intracellular ROS. The intracellular ROS activates the MAPK/NF-κB signaling resulting in IL-8 induction. This mechanism may possibly be at work to induce lung inflammation in mice.
Keywords: TRPA1; calcium; lipopolysaccharide; lung epithelial cell; lung inflammation; reactive oxygen species; signaling pathway.
Copyright © 2020 Ko, Lin, Perng, Lee and Kou.
Figures


Similar articles
-
Lung Epithelial TRPA1 Transduces the Extracellular ROS into Transcriptional Regulation of Lung Inflammation Induced by Cigarette Smoke: The Role of Influxed Ca²⁺.Mediators Inflamm. 2015;2015:148367. doi: 10.1155/2015/148367. Epub 2015 Oct 4. Mediators Inflamm. 2015. PMID: 26504357 Free PMC article.
-
Inflammatory Effects of Menthol vs. Non-menthol Cigarette Smoke Extract on Human Lung Epithelial Cells: A Double-Hit on TRPM8 by Reactive Oxygen Species and Menthol.Front Physiol. 2017 Apr 27;8:263. doi: 10.3389/fphys.2017.00263. eCollection 2017. Front Physiol. 2017. PMID: 28496415 Free PMC article.
-
Glucosamine attenuates cigarette smoke-induced lung inflammation by inhibiting ROS-sensitive inflammatory signaling.Free Radic Biol Med. 2014 Apr;69:208-18. doi: 10.1016/j.freeradbiomed.2014.01.026. Epub 2014 Jan 28. Free Radic Biol Med. 2014. PMID: 24486342
-
Lipopolysaccharide induces ICAM-1 expression via a c-Src/NADPH oxidase/ROS-dependent NF-κB pathway in human pulmonary alveolar epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2016 Apr 1;310(7):L639-57. doi: 10.1152/ajplung.00109.2014. Epub 2016 Jan 8. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 26747779
-
Transient Receptor Potential Ankyrin 1 (TRPA1) Channel as a Sensor of Oxidative Stress in Cancer Cells.Cells. 2023 Apr 26;12(9):1261. doi: 10.3390/cells12091261. Cells. 2023. PMID: 37174661 Free PMC article. Review.
Cited by
-
TRP Channels in Pulmonary Fibrosis: Variety Is a Spice of Life.Am J Respir Cell Mol Biol. 2023 Mar;68(3):241-242. doi: 10.1165/rcmb.2022-0446ED. Am J Respir Cell Mol Biol. 2023. PMID: 36413749 Free PMC article. No abstract available.
-
TRPA1 activation and Hsp90 inhibition synergistically downregulate macrophage activation and inflammatory responses in vitro.BMC Immunol. 2023 Jun 30;24(1):16. doi: 10.1186/s12865-023-00549-0. BMC Immunol. 2023. PMID: 37391696 Free PMC article.
-
The regulation of TRPA1 expression and function by Th1 and Th2-type inflammation in human A549 lung epithelial cells.Inflamm Res. 2023 Jul;72(7):1327-1339. doi: 10.1007/s00011-023-01750-y. Epub 2023 Jun 29. Inflamm Res. 2023. PMID: 37386145 Free PMC article.
-
Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use.Molecules. 2022 Aug 26;27(17):5481. doi: 10.3390/molecules27175481. Molecules. 2022. PMID: 36080253 Free PMC article. Review.
-
Voltage-gated ion channels are expressed in the Malpighian tubules and anal papillae of the yellow fever mosquito (Aedes aegypti), and may regulate ion transport during salt and water imbalance.J Exp Biol. 2024 Feb 1;227(3):jeb246486. doi: 10.1242/jeb.246486. Epub 2024 Feb 8. J Exp Biol. 2024. PMID: 38197515
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

