Short-term Cudrania tricuspidata fruit vinegar administration attenuates obesity in high-fat diet-fed mice by improving fat accumulation and metabolic parameters
- PMID: 33273564
- PMCID: PMC7712837
- DOI: 10.1038/s41598-020-78166-9
Short-term Cudrania tricuspidata fruit vinegar administration attenuates obesity in high-fat diet-fed mice by improving fat accumulation and metabolic parameters
Abstract
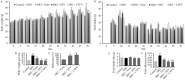
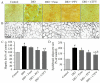
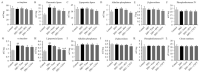
Previous studies have suggested that vinegar intake can help to reduce body fat and hyperglycemia. Therefore, this study aimed to evaluate the anti-obesity efficacy of vinegar fermented using Cudrania tricuspidata fruits (CTFV) and its main phenolic constituents and to analyze its molecular mechanism and changes in obesity-related metabolizing enzymatic activities. We found that HFD significantly caused hepatic steatosis; increases in body fats, feed efficiency, liver mass, lipids, insulin, oxidative parameters, cardiovascular-associated risk indices, lipase and α-amylase activities, whereas CTFV efficaciously attenuated HFD-induced oxidant stress, fat accumulation, obesity-related enzymatic activity, and the activation or reduction of obesity-related molecular reactions via improving metabolic parameters including phosphorylated insulin receptor substrate 1, protein tyrosine phosphatase 1B, phosphorylated phosphoinositide 3-kinase/protein kinase B, phosphorylated mitogen-activated protein kinases, sterol regulatory element-binding protein 1c, CCAAT/enhancer-binding protein, and fatty acid synthase; and decreases in adiponectin receptor 1, leptin receptor, adenosine monophosphate-activated protein kinase, acetyl-CoA carboxylase, and peroxisome proliferator-activated receptor, subsequently ameliorating HFD-induced obesity. Therefore, CTFV might provide a functional food resource or nutraceutical product for reducing body fat accumulation.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Anti-Obesity Effect of 6,8-Diprenylgenistein, an Isoflavonoid of Cudrania tricuspidata Fruits in High-Fat Diet-Induced Obese Mice.Nutrients. 2015 Dec 15;7(12):10480-90. doi: 10.3390/nu7125544. Nutrients. 2015. PMID: 26694457 Free PMC article.
-
A Combination of Apple Vinegar Drink with Bacillus coagulans Ameliorates High Fat Diet-Induced Body Weight Gain, Insulin Resistance and Hepatic Steatosis.Nutrients. 2020 Aug 19;12(9):2504. doi: 10.3390/nu12092504. Nutrients. 2020. PMID: 32825073 Free PMC article.
-
Effect of fruit vinegars on liver damage and oxidative stress in high-fat-fed rats.Pharm Biol. 2016;54(2):260-5. doi: 10.3109/13880209.2015.1031910. Epub 2015 Apr 8. Pharm Biol. 2016. PMID: 25853952
-
Antiobesity Effects of Salvia plebeia R. Br. Extract in High-Fat Diet-Induced Obese Mice.J Med Food. 2016 Nov;19(11):1048-1056. doi: 10.1089/jmf.2016.3763. Epub 2016 Oct 5. J Med Food. 2016. PMID: 27705068
-
Novel insights of dietary polyphenols and obesity.J Nutr Biochem. 2014 Jan;25(1):1-18. doi: 10.1016/j.jnutbio.2013.09.001. J Nutr Biochem. 2014. PMID: 24314860 Free PMC article. Review.
Cited by
-
Anti-Obesity and Anti-Hyperglycemic Effects of Meretrix lusoria Protamex Hydrolysate in ob/ob Mice.Int J Mol Sci. 2022 Apr 5;23(7):4015. doi: 10.3390/ijms23074015. Int J Mol Sci. 2022. PMID: 35409375 Free PMC article.
-
Antarctic Krill Euphausia superba Oil Supplementation Attenuates Hypercholesterolemia, Fatty Liver, and Oxidative Stress in Diet-Induced Obese Mice.Nutrients. 2024 Oct 24;16(21):3614. doi: 10.3390/nu16213614. Nutrients. 2024. PMID: 39519447 Free PMC article.
-
Deciphering the mechanism of jujube vinegar on hyperlipoidemia through gut microbiome based on 16S rRNA, BugBase analysis, and the stamp analysis of KEEG.Front Nutr. 2023 May 19;10:1160069. doi: 10.3389/fnut.2023.1160069. eCollection 2023. Front Nutr. 2023. PMID: 37275638 Free PMC article.
-
Short-Term Effects of PJE Administration on Metabolic Parameters in Diet-Induced Obesity Mice.Foods. 2023 Apr 17;12(8):1675. doi: 10.3390/foods12081675. Foods. 2023. PMID: 37107470 Free PMC article.
-
Systemic Review of Clot Retraction Modulators.Int J Mol Sci. 2023 Jun 25;24(13):10602. doi: 10.3390/ijms241310602. Int J Mol Sci. 2023. PMID: 37445780 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

