Lmx1a and Lmx1b are Redundantly Required for the Development of Multiple Components of the Mammalian Auditory System
- PMID: 33246067
- PMCID: PMC7780644
- DOI: 10.1016/j.neuroscience.2020.11.013
Lmx1a and Lmx1b are Redundantly Required for the Development of Multiple Components of the Mammalian Auditory System
Abstract
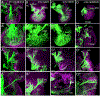
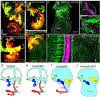
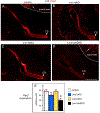
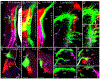
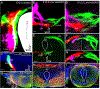
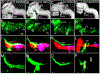
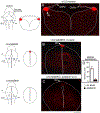
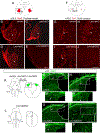
The inner ear, projections, and brainstem nuclei are essential components of the auditory and vestibular systems. It is believed that the evolution of complex systems depends on duplicated sets of genes. The contribution of duplicated genes to auditory or vestibular system development, however, is poorly understood. We describe that Lmx1a and Lmx1b, which originate from the invertebrate Lmx1b-like gene, redundantly regulate development of multiple essential components of the mammalian auditory/vestibular systems. Combined, but not individual, loss of Lmx1a/b eliminated the auditory inner ear organ of Corti (OC) and disrupted the spiral ganglion, which was preceded by a diminished expression of their critical regulator Pax2. Innervation of the remaining inner ear vestibular organs revealed unusual sizes or shapes and was more affected compared to Lmx1a/b single-gene mutants. Individual loss of Lmx1a/b genes did not disrupt brainstem auditory nuclei or inner ear central projections. Combined loss of Lmx1a/b, however, eliminated excitatory neurons in cochlear/vestibular nuclei, and also the expression of a master regulator Atoh1 in their progenitors in the lower rhombic lip (RL). Finally, in Lmx1a/b double mutants, vestibular afferents aberrantly projected to the roof plate. This phenotype was associated with altered expression of Wnt3a, a secreted ligand of the Wnt pathway that regulates pathfinding of inner ear projections. Thus, Lmx1a/b are redundantly required for the development of the mammalian inner ear, inner ear central projections, and cochlear/vestibular nuclei.
Keywords: LIM-homeodomain transcription factors; auditory system; ear central projections; hindbrain; neurosensory development; roof plate.
Copyright © 2020 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


Similar articles
-
Molecular mechanisms governing development of the hindbrain choroid plexus and auditory projection: A validation of the seminal observations of Wilhelm His.IBRO Neurosci Rep. 2022 Oct 3;13:306-313. doi: 10.1016/j.ibneur.2022.09.011. eCollection 2022 Dec. IBRO Neurosci Rep. 2022. PMID: 36247525 Free PMC article. Review.
-
Reciprocal Negative Regulation Between Lmx1a and Lmo4 Is Required for Inner Ear Formation.J Neurosci. 2018 Jun 6;38(23):5429-5440. doi: 10.1523/JNEUROSCI.2484-17.2018. Epub 2018 May 16. J Neurosci. 2018. PMID: 29769265 Free PMC article.
-
Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis.Cell Tissue Res. 2008 Dec;334(3):339-58. doi: 10.1007/s00441-008-0709-2. Epub 2008 Nov 5. Cell Tissue Res. 2008. PMID: 18985389 Free PMC article.
-
Development in the Mammalian Auditory System Depends on Transcription Factors.Int J Mol Sci. 2021 Apr 18;22(8):4189. doi: 10.3390/ijms22084189. Int J Mol Sci. 2021. PMID: 33919542 Free PMC article. Review.
-
Mutanlallemand (mtl) and Belly Spot and Deafness (bsd) are two new mutations of Lmx1a causing severe cochlear and vestibular defects.PLoS One. 2012;7(11):e51065. doi: 10.1371/journal.pone.0051065. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226461 Free PMC article.
Cited by
-
Molecular mechanisms governing development of the hindbrain choroid plexus and auditory projection: A validation of the seminal observations of Wilhelm His.IBRO Neurosci Rep. 2022 Oct 3;13:306-313. doi: 10.1016/j.ibneur.2022.09.011. eCollection 2022 Dec. IBRO Neurosci Rep. 2022. PMID: 36247525 Free PMC article. Review.
-
Shared and organ-specific gene-expression programs during the development of the cochlea and the superior olivary complex.RNA Biol. 2023 Jan;20(1):629-640. doi: 10.1080/15476286.2023.2247628. RNA Biol. 2023. PMID: 37602850 Free PMC article.
-
Molecular Organization and Patterning of the Medulla Oblongata in Health and Disease.Int J Mol Sci. 2022 Aug 17;23(16):9260. doi: 10.3390/ijms23169260. Int J Mol Sci. 2022. PMID: 36012524 Free PMC article. Review.
-
A human induced pluripotent stem cell-based modular platform to challenge sensorineural hearing loss.Stem Cells. 2021 Jun;39(6):697-706. doi: 10.1002/stem.3346. Epub 2021 Feb 8. Stem Cells. 2021. PMID: 33522002 Free PMC article. Review.
-
Sensorineural Hearing Loss and Mitochondrial Apoptosis of Cochlear Spiral Ganglion Neurons in Fibroblast Growth Factor 13 Knockout Mice.Front Cell Neurosci. 2021 Jun 16;15:658586. doi: 10.3389/fncel.2021.658586. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34220452 Free PMC article.
References
-
- Abello G, Khatri S, Radosevic M, Scotting P, Giraldez F, Alsina B (2010) Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Developmental Biology 339:166–178. - PubMed
-
- Boëda B, El-Amraoui A, Bahloul A, Goodyear R, Daviet L, Blanchard S, Perfettini I, Fath KR, Shorte S, Reiners J, Houdusse A, Legrain P, Wolfrum U, Richardson G, Petit C (2002) Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. EMBO J 21:6689–99. - PMC - PubMed
-
- Butts T, Green MJ, Wingate RJ (2014) Development of the cerebellum: simple steps to make a ‘little brain’. Development 141:4031–4041. - PubMed
-
- Chang C-H, Tsai R-K, Tsai M-H, Lin Y-H, Hirobe T (2014) The roles of Frizzled-3 and Wnt3a on melanocyte development: in vitro studies on neural crest cells and melanocyte precursor cell lines. Journal of Dermatological Science 75:100–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

