Roles of ten-eleven translocation family proteins and their O-linked β-N-acetylglucosaminylated forms in cancer development
- PMID: 33240407
- PMCID: PMC7681232
- DOI: 10.3892/ol.2020.12262
Roles of ten-eleven translocation family proteins and their O-linked β-N-acetylglucosaminylated forms in cancer development
Abstract
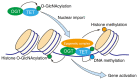
Members of the ten-eleven translocation (TET) protein family of which three mammalian TET proteins have been discovered so far, catalyze the sequential oxidation of 5-methylcytosine to 5-hydroxymethylcytosine, 5-formylcytosine, and 5-carboxylcytosine which serve an important role in embryonic development and tumor progression. O-GlcNAcylation (O-linked β-N-acetylglucosaminylation) is a reversible post-translational modification known to serve important roles in tumorigenesis and metastasis especially in hematopoietic malignancies such as myelodysplastic syndromes, chronic myelomonocytic leukemia and acute myeloid leukemia. O-GlcNAcylation activity requires only two enzymes: O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA). OGT catalyzes attachment of GlcNAc sugar to serine, threonine and cytosine residues in proteins, while OGA hydrolyzes O-GlcNAc attached to proteins. Numerous recent studies have demonstrated that TETs can be O-GlcNAcylated by OGT, with consequent alteration of TET activity and stability. The present review focuses on the cellular, biological and biochemical functions of TET and its O-GlcNAcylated form and proposes a model of the role of TET/OGT complex in regulation of target proteins during cancer development. In addition, the present review provides directions for future research in this area.
Keywords: O-GlcNAcylation; TET.
Copyright: © Li et al.
Figures

Similar articles
-
Differential regulation of the ten-eleven translocation (TET) family of dioxygenases by O-linked β-N-acetylglucosamine transferase (OGT).J Biol Chem. 2014 Feb 28;289(9):5986-96. doi: 10.1074/jbc.M113.524140. Epub 2014 Jan 6. J Biol Chem. 2014. PMID: 24394411 Free PMC article.
-
O-GlcNAcylation: A New Cancer Hallmark?Front Endocrinol (Lausanne). 2013 Aug 12;4:99. doi: 10.3389/fendo.2013.00099. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 23964270 Free PMC article.
-
Phosphorylation of TET proteins is regulated via O-GlcNAcylation by the O-linked N-acetylglucosamine transferase (OGT).J Biol Chem. 2015 Feb 20;290(8):4801-4812. doi: 10.1074/jbc.M114.605881. Epub 2015 Jan 7. J Biol Chem. 2015. PMID: 25568311 Free PMC article.
-
O-GlcNAc transferase inhibitors: current tools and future challenges.Biochem Soc Trans. 2016 Feb;44(1):88-93. doi: 10.1042/BST20150189. Biochem Soc Trans. 2016. PMID: 26862193 Review.
-
The roles of OGT and its mechanisms in cancer.Cell Biosci. 2024 Sep 16;14(1):121. doi: 10.1186/s13578-024-01301-w. Cell Biosci. 2024. PMID: 39285476 Free PMC article. Review.
Cited by
-
The effect of gallic acid on memory and anxiety-like behaviors in rats with bile duct ligation-induced hepatic encephalopathy: Role of AMPK pathway.Avicenna J Phytomed. 2022 Jul-Aug;12(4):425-438. doi: 10.22038/AJP.2022.19720. Avicenna J Phytomed. 2022. PMID: 35782765 Free PMC article.
-
Structure and Function of TET Enzymes.Adv Exp Med Biol. 2022;1389:239-267. doi: 10.1007/978-3-031-11454-0_10. Adv Exp Med Biol. 2022. PMID: 36350513
-
O-GlcNAcylation: an important post-translational modification and a potential therapeutic target for cancer therapy.Mol Med. 2022 Sep 14;28(1):115. doi: 10.1186/s10020-022-00544-y. Mol Med. 2022. PMID: 36104770 Free PMC article. Review.
-
Tracing TET1 expression in prostate cancer: discovery of malignant cells with a distinct oncogenic signature.Clin Epigenetics. 2021 Nov 29;13(1):211. doi: 10.1186/s13148-021-01201-7. Clin Epigenetics. 2021. PMID: 34844636 Free PMC article.
-
O-GlcNAcylation and O-GlcNAc Cycling Regulate Gene Transcription: Emerging Roles in Cancer.Cancers (Basel). 2021 Apr 1;13(7):1666. doi: 10.3390/cancers13071666. Cancers (Basel). 2021. PMID: 33916244 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
