Functionally distinct resident macrophage subsets differentially shape responses to infection in the bladder
- PMID: 33239294
- PMCID: PMC7688323
- DOI: 10.1126/sciadv.abc5739
Functionally distinct resident macrophage subsets differentially shape responses to infection in the bladder
Abstract
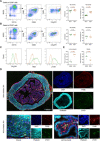
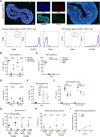
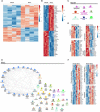
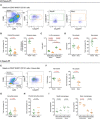
Resident macrophages are abundant in the bladder, playing key roles in immunity to uropathogens. Yet, whether they are heterogeneous, where they come from, and how they respond to infection remain largely unknown. We identified two macrophage subsets in mouse bladders, MacM in muscle and MacL in the lamina propria, each with distinct protein expression and transcriptomes. Using a urinary tract infection model, we validated our transcriptomic analyses, finding that MacM macrophages phagocytosed more bacteria and polarized to an anti-inflammatory profile, whereas MacL macrophages died rapidly during infection. During resolution, monocyte-derived cells contributed to tissue-resident macrophage pools and both subsets acquired transcriptional profiles distinct from naïve macrophages. Macrophage depletion resulted in the induction of a type 1-biased immune response to a second urinary tract infection, improving bacterial clearance. Our study uncovers the biology of resident macrophages and their responses to an exceedingly common infection in a largely overlooked organ, the bladder.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


Similar articles
-
Timing is everything: impact of development, ageing and circadian rhythm on macrophage functions in urinary tract infections.Mucosal Immunol. 2022 Jun;15(6):1114-1126. doi: 10.1038/s41385-022-00558-z. Epub 2022 Aug 29. Mucosal Immunol. 2022. PMID: 36038769 Review.
-
Uropathogenic Escherichia coli Engages CD14-Dependent Signaling to Enable Bladder-Macrophage-Dependent Control of Acute Urinary Tract Infection.J Infect Dis. 2016 Feb 15;213(4):659-68. doi: 10.1093/infdis/jiv424. Epub 2015 Aug 30. J Infect Dis. 2016. PMID: 26324782
-
Bladder resident macrophages: Mucosal sentinels.Cell Immunol. 2018 Aug;330:136-141. doi: 10.1016/j.cellimm.2018.01.018. Epub 2018 Feb 2. Cell Immunol. 2018. PMID: 29422271 Review.
-
Simian Immunodeficiency Virus Targeting of CXCR3+ CD4+ T Cells in Secondary Lymphoid Organs Is Associated with Robust CXCL10 Expression in Monocyte/Macrophage Subsets.J Virol. 2017 Jun 9;91(13):e00439-17. doi: 10.1128/JVI.00439-17. Print 2017 Jul 1. J Virol. 2017. PMID: 28424283 Free PMC article.
-
Macrophages Subvert Adaptive Immunity to Urinary Tract Infection.PLoS Pathog. 2015 Jul 16;11(7):e1005044. doi: 10.1371/journal.ppat.1005044. eCollection 2015 Jul. PLoS Pathog. 2015. PMID: 26182347 Free PMC article.
Cited by
-
Nur77 protects the bladder urothelium from intracellular bacterial infection.Nat Commun. 2024 Sep 27;15(1):8308. doi: 10.1038/s41467-024-52454-8. Nat Commun. 2024. PMID: 39333075 Free PMC article.
-
Polychlorinated biphenyl (PCB) exposure in adult female mice can influence bladder contractility.Am J Clin Exp Urol. 2023 Oct 15;11(5):367-384. eCollection 2023. Am J Clin Exp Urol. 2023. PMID: 37941647 Free PMC article.
-
Probiotic Limosilactobacillus reuteri KUB-AC5 decreases urothelial cell invasion and enhances macrophage killing of uropathogenic Escherichia coli in vitro study.Front Cell Infect Microbiol. 2024 Jul 18;14:1401462. doi: 10.3389/fcimb.2024.1401462. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39091675 Free PMC article.
-
Multimodal Single-Cell Analyses Outline the Immune Microenvironment and Therapeutic Effectors of Interstitial Cystitis/Bladder Pain Syndrome.Adv Sci (Weinh). 2022 Jun;9(18):e2106063. doi: 10.1002/advs.202106063. Epub 2022 Apr 25. Adv Sci (Weinh). 2022. PMID: 35470584 Free PMC article.
-
Timing is everything: impact of development, ageing and circadian rhythm on macrophage functions in urinary tract infections.Mucosal Immunol. 2022 Jun;15(6):1114-1126. doi: 10.1038/s41385-022-00558-z. Epub 2022 Aug 29. Mucosal Immunol. 2022. PMID: 36038769 Review.
References
-
- Amit I., Winter D. R., Jung S., The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat. Immunol. 17, 18–25 (2016). - PubMed
-
- Ingersoll M. A., Albert M. L., From infection to immunotherapy: Host immune responses to bacteria at the bladder mucosa. Mucosal Immunol. 6, 1041–1053 (2013). - PubMed
-
- Lacerda Mariano L., Ingersoll M. A., Bladder resident macrophages: Mucosal sentinels. Cell. Immunol. 330, 136–141 (2018). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

